VOLUMEN- barium sulfate suspension
E-Z-EM Canada Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
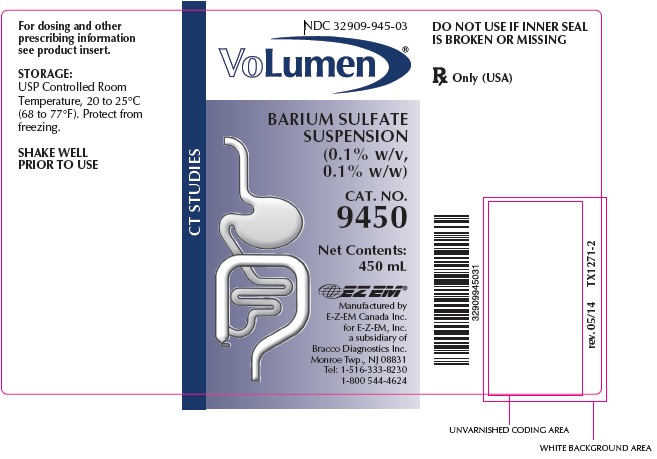
NDC 32909-945-03
VOLUMEN®
BARIUM SULFATE SUSPENSION
(0.1% w/v, 0.1%
w/w)
DESCRIPTION
VoLumen® is a barium sulfate suspension 0.1% w/v, 0.1% w/w for oral administration. Each 100 mL contains 0.1 g barium sulfate. Barium sulfate, due to its high molecular density is opaque to x-rays and therefore, acts as a positive contrast agent for radiographic studies. The active ingredient is barium sulfate and its structural formula is BaSO4. Barium sulfate occurs as a fine, white, odorless, tasteless, bulky powder which is free from grittiness. Its aqueous suspensions are neutral to litmus. It is practically insoluble in water, solutions of acids and alkalies, and organic solvents.
CLINICAL PHARMACOLOGY
Barium sulfate, due to its high molecular density is opaque to x-rays and, therefore, acts as a positive contrast agent for radiographic studies. Barium sulfate is biologically inert and, therefore, is not absorbed or metabolized by the body, and is eliminated from the GI tract unchanged.
CONTRAINDICATIONS
This product should not be used in patients with known or suspected gastrointestinal perforation or hypersensitivity to barium sulfate or any component of this barium sulfate formulation.
WARNINGS
Rarely, severe allergic reactions of an anaphylactoid nature, have been reported following administration of barium sulfate contrast agents. Appropriately trained personnel and facilities should be available for emergency treatment of severe reactions and should remain available for at least 30 to 60 minutes following administration, since delayed reactions can occur.
PRECAUTIONS
General
Diagnostic procedures which involve the use of radiopaque contrast agents should be carried out under the direction of personnel with the requisite training and with a thorough knowledge of the particular procedure to be performed. A history of bronchial asthma, atopy, as evidenced by hay fever and eczema, or a previous reaction to a contrast agent, warrant special attention. Caution should be exercised with the use of radiopaque media in severely debilitated patients and in those with marked hypertension or advanced cardiac disease. Ingestion of barium is not recommended in patients with a history of food aspiration. If barium studies are required in these patients or in patients in whom integrity of the swallowing mechanism is unknown, proceed with caution. If barium is aspirated into the larynx, further administration should be immediately discontinued.
Information for Patients
Before administration of this product, patients receiving barium sulfate diagnostic agents should be instructed to:
- Inform their physician if they are pregnant.
- Inform their physician if they are allergic to any drugs or food, or if they have had any prior reactions to barium sulfate products or other contrast agents used in x-ray procedures (see PRECAUTIONS-General).
- Inform their physician about any other medications they are currently taking.
- Seek immediate medical attention if they experience an allergic reaction after using this product.
Drug Interactions
The presence of barium sulfate formulations in the GI tract may alter the absorption of therapeutic agents taken concomitantly. In order to minimize any potential change in absorption, the separate administration of barium sulfate from that of other agents should be considered.
ADVERSE REACTIONS
Adverse reactions, such as nausea, vomiting, diarrhea and abdominal cramping, accompanying the use of barium sulfate formulations are infrequent and usually mild. Severe reactions (approximately 1 in 1,000,000) and fatalities (approximately 1 in 10,000,000) have occurred. Procedural complications are rare, but may include aspiration pneumonitis, granuloma formation, intravasation, embolization and peritonitis following intestinal perforation, vasovagal and syncopal episodes, and fatalities.
ALLERGIC REACTIONS
Due to the increased likelihood of allergic reactions in atopic patients, it is important that a complete history of known and suspected allergies as well as allergic-like symptoms, e.g., rhinitis, bronchial asthma, eczema and urticaria, be obtained prior to any medical procedure utilizing these products. A mild allergic reaction would most likely include generalized pruritus, erythema or urticaria (approximately 1 in 250,000). Such reactions will generally respond to an antihistamine such as 50 mg of diphenhydramine or its equivalent. In the rarer, more serious reactions (approximately 1 in 1,000,000) laryngeal edema, bronchospasm or hypotension could develop. Severe reactions which may require emergency measures are often characterized by peripheral vasodilation, hypotension, reflex tachycardia, dyspnea, agitation, confusion and cyanosis, progressing to unconsciousness. Treatment should be initiated immediately with 0.3 to 0.5 cc of 1:1000 epinephrine subcutaneously. If bronchospasm predominates, 0.25 to 0.50 grams of intravenous aminophylline should be given slowly. Appropriate vasopressors might be required. Adrenocorticosteroids, even if given intravenously, exert no significant effect on the acute allergic reactions for a few hours. The administration of these agents should not be regarded as emergency measures for the treatment of allergic reactions. Apprehensive patients may develop weakness, pallor, tinnitus, diaphoresis and bradycardia following the administration of any diagnostic agent. Such reactions are usually non-allergic in nature and are best treated by having the patient lie flat for an additional 10 to 30 minutes under observation.
OVERDOSAGE
On rare occasions following repeated administration, severe stomach cramps, nausea, vomiting, diarrhea or constipation may occur. These indicated responses can be present in both fluoroscopic and CT procedures. These are transitory in nature and are not considered serious. Symptoms may be treated according to currently accepted standards of medical care.
DOSAGE AND ADMINISTRATION
The volume of the CT barium sulfate suspension to be administered will depend on the degree and extent of contrast required in the area(s) under examination and on the technique employed.
For Oral Administration: GI Tract Marking
The patient should begin drinking VoLumen® approximately 20 – 30 minutes prior to the scheduled procedure. It is recommended that the patient consume multiple bottles, about 900 mL to 1,350 mL total volume prior to the exam or use as directed by physician. For improved gastric marking have patient consume the final 200 mL immediately prior to scan. Bowel marking is consistent due to the uniformity of the 0.1% concentration of BaSO4. Bowel lumen marking can be improved by increasing the volume of VoLumen® consumed (see below). Other dosing regimens may be followed as applicable. In patients where marking is problematic, such as obesity and delayed transit, improved marking may be possible by increasing the total volume of VoLumen® administered up to 1,800 mL or four (4) bottles.
| VOLUMEN
barium sulfate suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - E-Z-EM Canada Inc (204211163) |
| Registrant - E-Z-EM, INC. (002041226) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| E-Z-EM Canada Inc | 204211163 | PACK(32909-945) , ANALYSIS(32909-945) , LABEL(32909-945) , MANUFACTURE(32909-945) | |