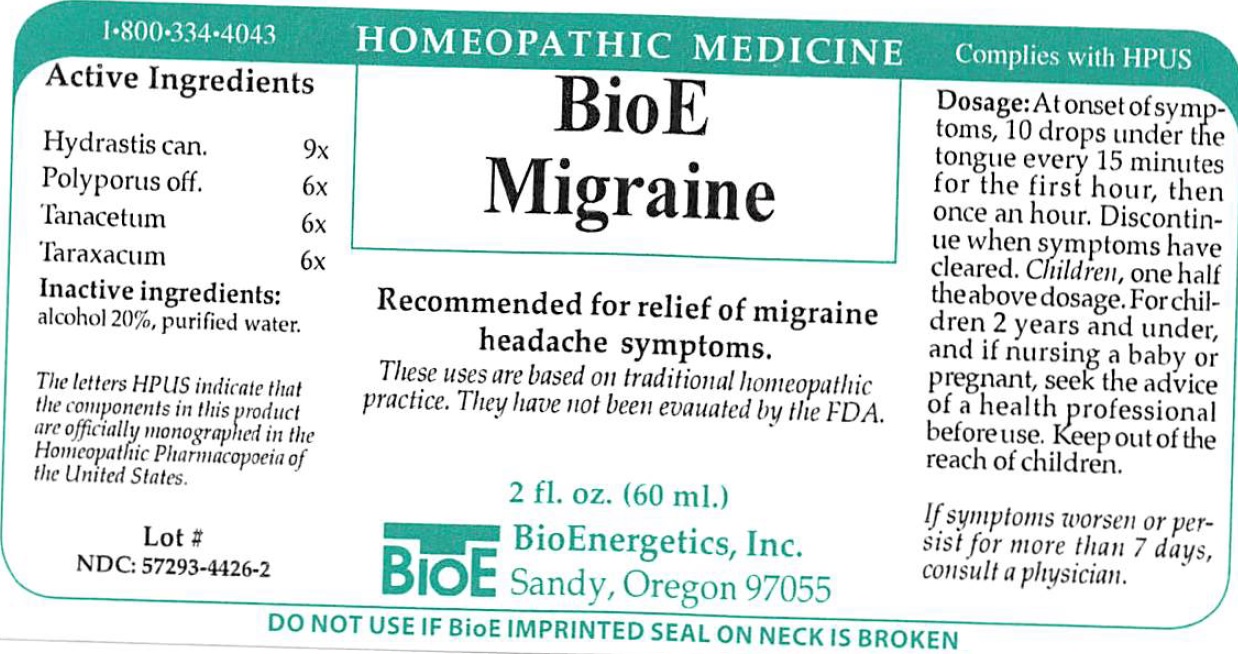
BIOE MIGRAINE- goldenseal, laricifomes officinalis fruiting body, tanacetum vulgare top, taraxacum palustre root, liquid
BioEnergetics, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
BioE Migraine
Recommended for relief of migraine headache symptoms.
These uses are based on traditional homeopathic practice, They have not been evauated by the FDA.
Dosage:
At onset of symptoms, 10 drops under the tongue every 15 minutes for the first hour, then once an hour. Discontinue when symptoms have cleared. Children, one half the above dosage. For children 2 years and under, and if nursing a baby or pregnant, seek the advice of a health professional before use.
| BIOE MIGRAINE
goldenseal, laricifomes officinalis fruiting body, tanacetum vulgare top, taraxacum palustre root, liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - BioEnergetics, Inc. (102847014) |
| Registrant - BioEnergetics, Inc. (102847014) |