BASIC CASE HYDROCORTISONE ANTI-ITCH- hydrocortisone cream
L. Perrigo Company
----------
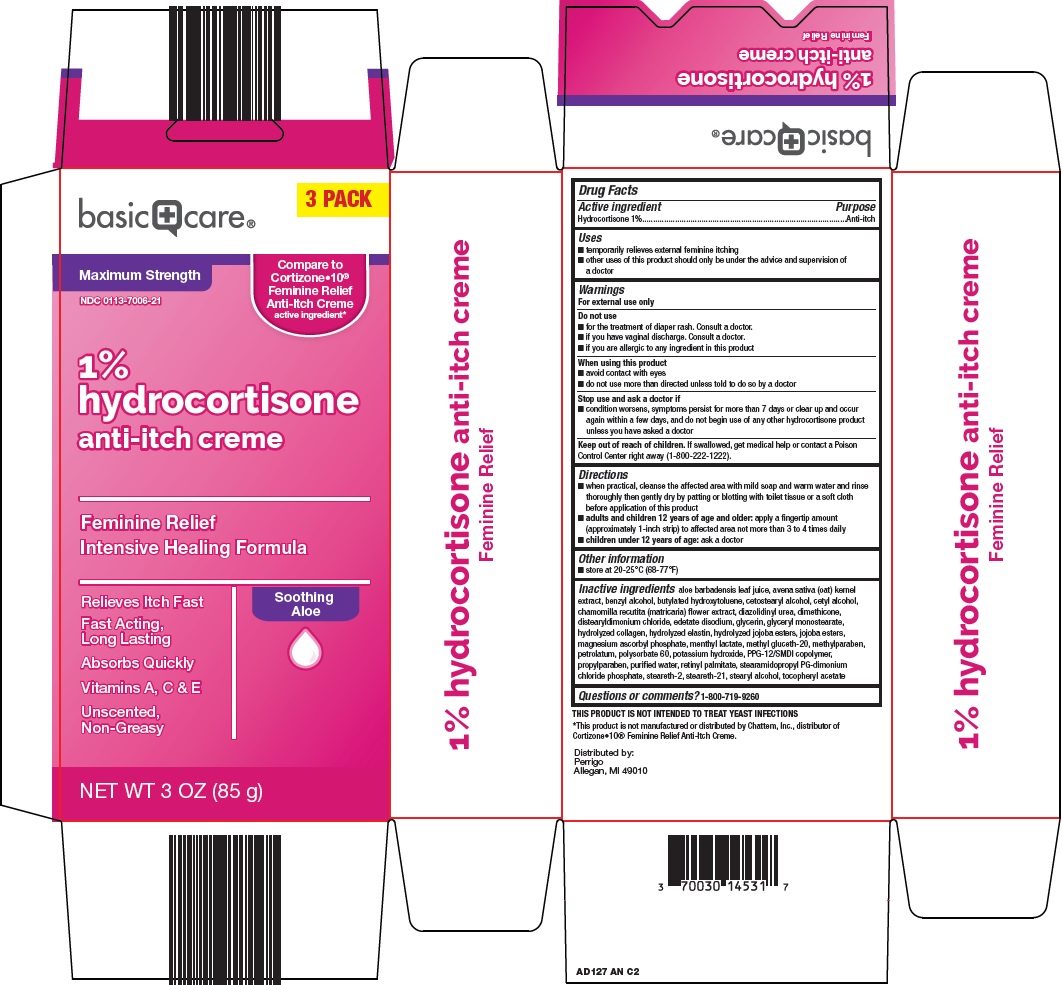
Amazon 1% Hydrocortisone Anti-Itch Creme Drug Facts
Uses
- •
- temporarily relieves external feminine itching
- •
- other uses of this product should only be under the advice and supervision of a doctor
Do not use
- •
- for the treatment of diaper rash. Consult a doctor.
- •
- if you have vaginal discharge. Consult a doctor.
- •
- if you are allergic to any ingredient in this product
When using this product
- •
- avoid contact with eyes
- •
- do not use more than directed unless told to do so by a doctor
Stop use and ask a doctor if
- •
- condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days, and do not begin use of any other hydrocortisone product unless you have asked a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- •
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly then gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
- •
- adults and children 12 years of age and older: apply a fingertip amount (approximately 1-inch strip) to affected area not more than 3 to 4 times daily
- •
- children under 12 years of age: ask a doctor
Inactive ingredients
aloe barbadensis leaf juice, avena sativa (oat) kernel extract, benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, cetyl alcohol, chamomilla recutita (matricaria) flower extract, diazolidinyl urea, dimethicone, distearyldimonium chloride, edetate disodium, glycerin, glyceryl monostearate, hydrolyzed collagen, hydrolyzed elastin, hydrolyzed jojoba esters, jojoba esters, magnesium ascorbyl phosphate, menthyl lactate, methyl gluceth-20, methylparaben, petrolatum, polysorbate 60, potassium hydroxide, PPG-12/SMDI copolymer, propylparaben, purified water, retinyl palmitate, stearamidopropyl PG-dimonium chloride phosphate, steareth-2, steareth-21, stearyl alcohol, tocopheryl acetate
Package/Label Principal Display Panel
3 PACK
Maximum Strength
Compare to Cortizone•10® Feminine Relief Anti-Itch Creme active ingredient
1%
hydrocortisone
anti-itch creme
Feminine Relief
Intensive Healing Formula
Relieves Itch Fast
Fast Acting, Long Lasting
Absorbs Quickly
Vitamins A, C & E
Unscented, Non-Greasy
Soothing Aloe
NET WT 3 OZ (85 g)
| BASIC CASE HYDROCORTISONE ANTI-ITCH
hydrocortisone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |