Label: ALLERGY RELIEF CHILDRENS ALLERGY- diphenhydramine hydrochloride liquid
- NDC Code(s): 70000-0492-1
- Packager: Cardinal Health (Leader) 70000
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 4, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
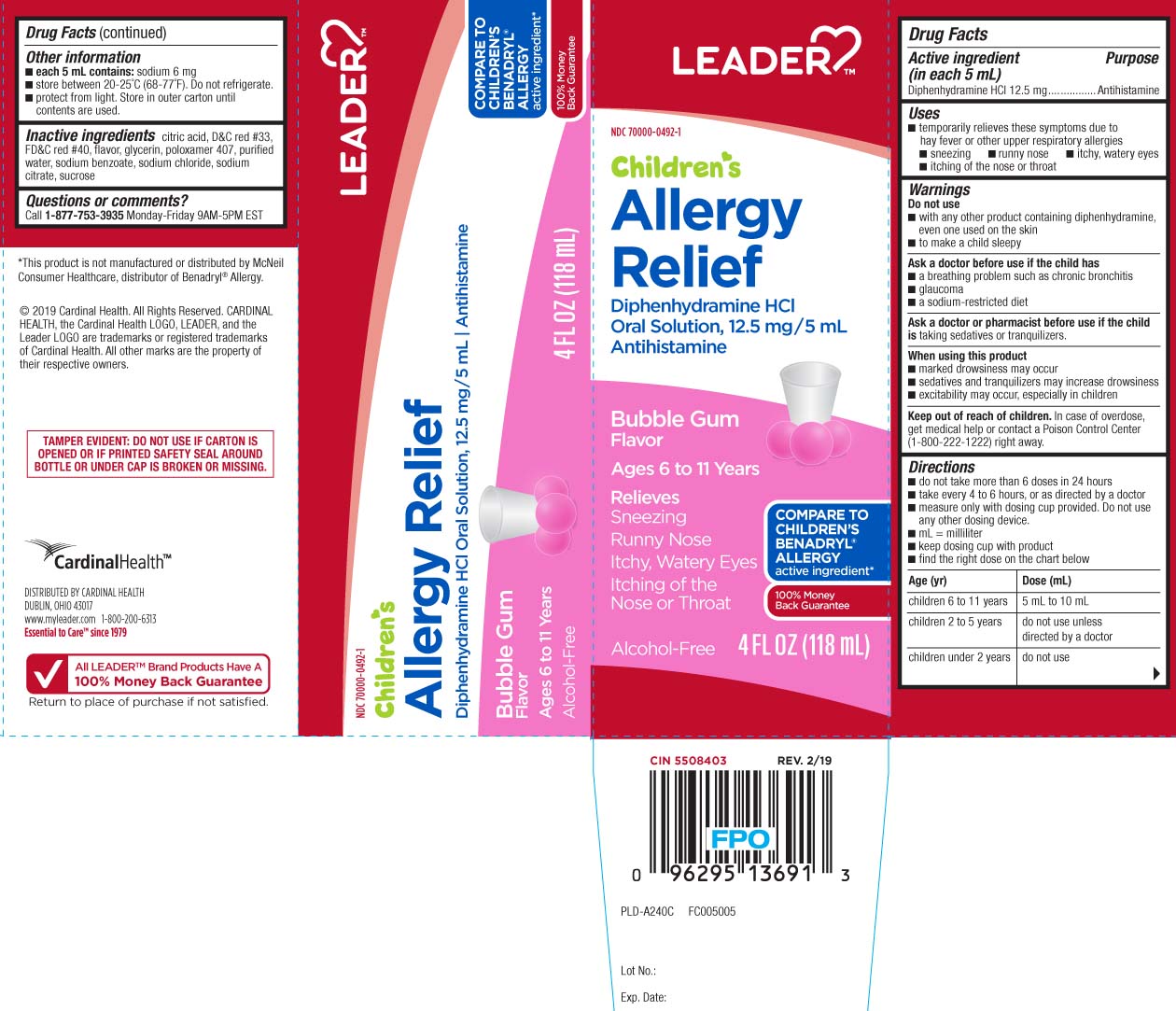
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if the child has
- a breathing problem such as chronic bronchitis
- glaucoma
- a sodium-restricted diet
-
Directions
- do not take more than 6 doses in 24 hours
- take every 4 to 6 hours, or as directed by a doctor
- measure only with dosing cup provided. Do not use any other dosing device.
- mL = milliliter
- keep dosing cup with product
- find the right dose on the chart below
age (yr) dose (mL) children 6 to 11 years 5 mL to 10 mL children 2 to 5 years do not use unless directed by a doctor children under 2 years do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO CHILDREN'S BENADRYL® ALLERGY active ingredient*
Children's
Allergy Relief
Diphenhydramine HCI
Oral Solution, 12.5 mg/5mL
Antihistamine
Bubble Gum Flavor
Ages 6 to 11 years
Relieves
Sneezing
Runny Nose
Itchy, Watery Eyes
Itching of the Nose or Throat
Alcohol-Free
Dosing Cup included
FL OZ (mL)
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Benadryl® Allergy
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF CHILDRENS ALLERGY
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0492 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) POLOXAMER 407 (UNII: TUF2IVW3M2) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0492-1 1 in 1 BOX 02/28/2019 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/28/2019 Labeler - Cardinal Health (Leader) 70000 (063997360)