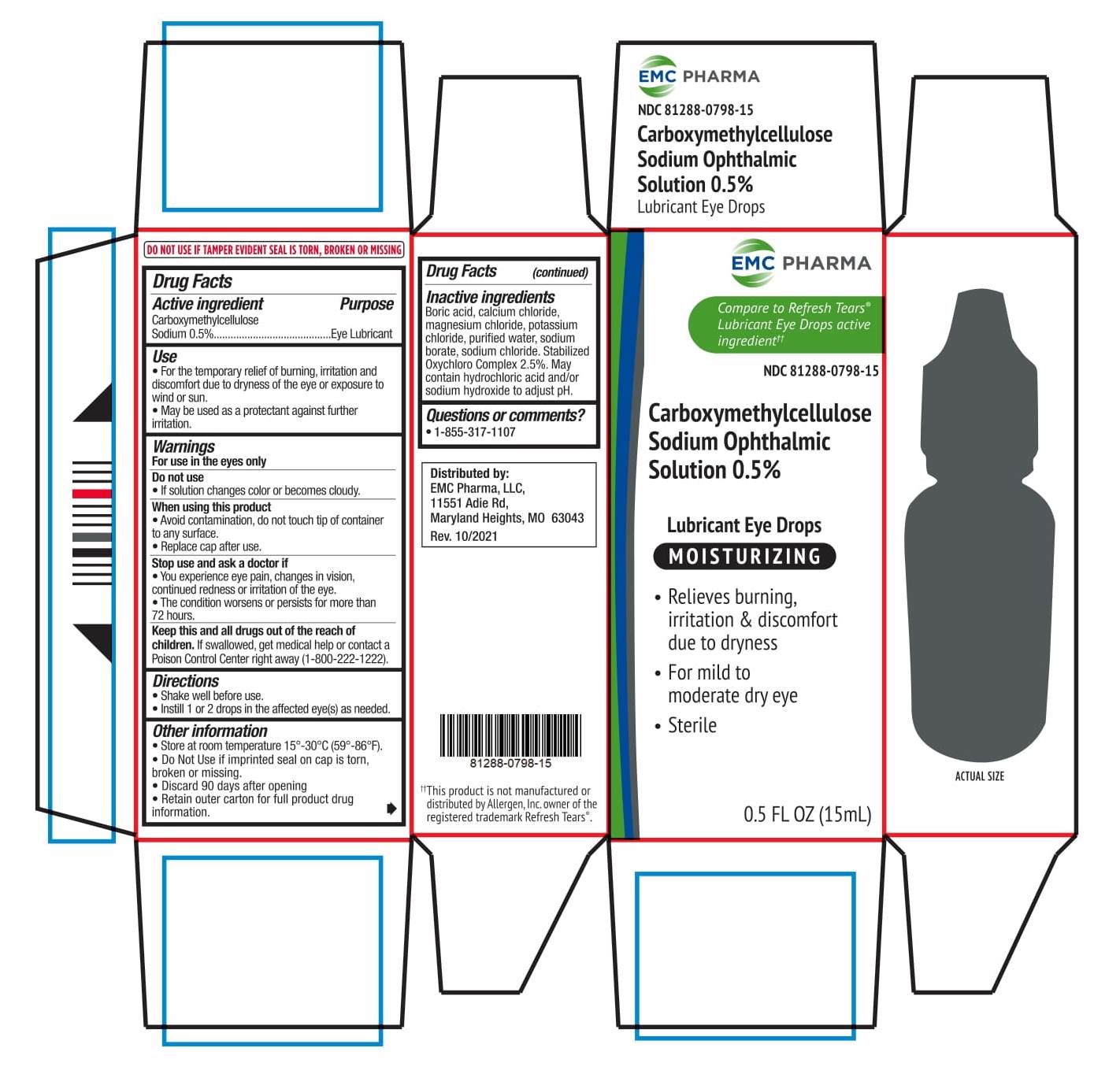
CARBOXYMETHYLCELLULOSE SODIUM OPHTHALMIC SOLUTION 0.5%- carboxymethylcellulose sodium solution/ drops
EMC Pharma, LLC
----------
Carboxymethylcellulose Sodium Ophthalmic Solution 0.5%
Use
• For the temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind or sun.
• May be used as protectant against further irritation.
When using this product
• Avoid contamination, do not touch tip of container to any surface.
• Replace cap after use.
Stop use and ask a doctor if
• You experience eye pain, changes in vision, continued redness or irritation of the eye.
• The condition worsens or persists for more than 72 hours
Keep this and all drugs out of the reach of the children. If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222)
Other Information
• Store at room temperature 15°- 30 ° (59 °- 86 °F).
• Do Not Use if imprinted seal on cap is torn, broken or missing.
• Discard 90 days after opening
• Retain outer carton for full product drug information
| CARBOXYMETHYLCELLULOSE SODIUM OPHTHALMIC SOLUTION 0.5%
carboxymethylcellulose sodium solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - EMC Pharma, LLC (117754047) |
| Registrant - EMC Pharma, LLC (117754047) |