HYPOTEARS- polyvinyl alcohol and polyehtylene glycol 400 solution/ drops
Novartis Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
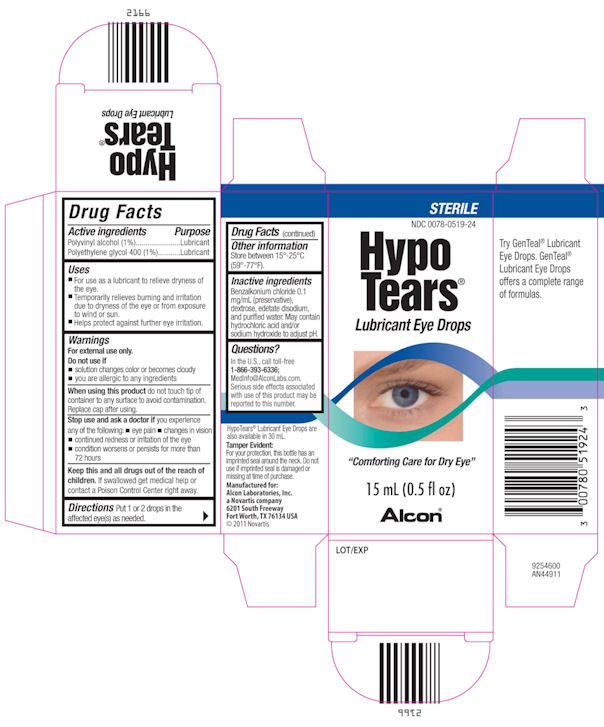
Drug Facts
INDICATIONS & USAGE SECTION
- For use as a lubricant to relieve dryness of the eye.
- Temporarily relieves burning and irritation due to dryness of the eye or from exposure to wind or sun.
- Helps protect against further eye irritation.
OTC - DO NOT USE SECTION
- if solution changes colors or becomes cloudy
- if you are allergic to any ingredients
OTC - WHEN USING SECTION
When using this product do not touch tip of container to any surface to avoid contamination.
Replace cap after using.
OTC - STOP USE SECTION
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condiiton worsens or persists for more than 72 hours
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep this and all drugs out of the reach of children. If swallowed get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT SECTION
Benzalkonium chloride 0.1 mg/mL (preservative), dextrose, edetate disodium, and purified water. May contain hydrochloric acid and/or sodium hydroxide to adjut pH.
OTC - QUESTIONS SECTION
In the U.S. call toll-free
1-866-393-6336;
MedInfo@AlconLabs.com.
Serious side effects associated with use of this product may be reported to this number.
| HYPOTEARS
polyvinyl alcohol and polyehtylene glycol 400 solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Novartis Pharmaceutical Corporation (002147023) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 079587625 | manufacture(0078-0519) | |