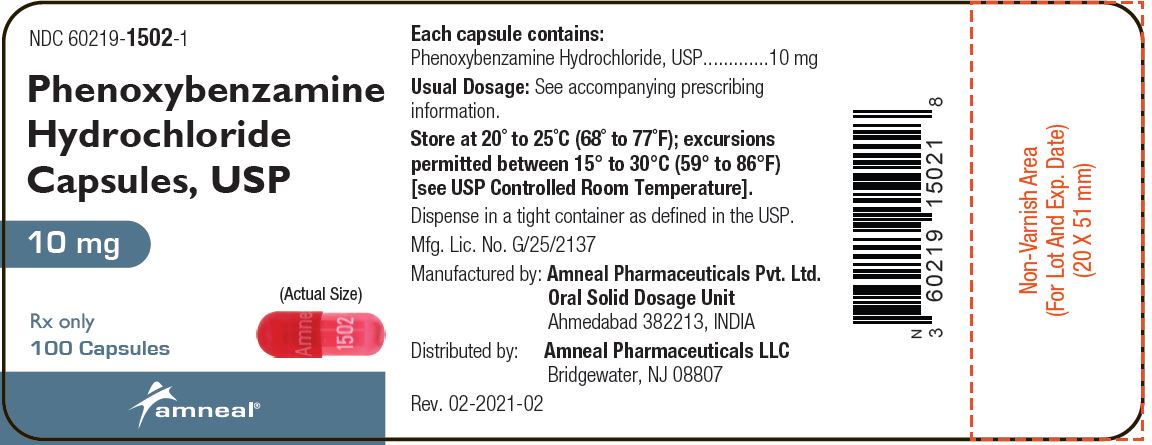
Label: PHENOXYBENZAMINE HYDROCHLORIDE capsule
- NDC Code(s): 60219-1502-1
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each phenoxybenzamine hydrochloride capsule, USP with red cap and body, is imprinted with “Amneal” on cap and “1502” on body, and contains 10 mg of phenoxybenzamine hydrochloride, USP. Inactive ingredients consist of colloidal silicon dioxide, D&C red 33, FD & C red 3, gelatin, iron oxide yellow, lactose monohydrate and sodium lauryl sulfate.
The capsule is imprinted with white pharmaceutical ink which contains butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac, strong ammonia solution and titanium dioxide.
Phenoxybenzamine hydrochloride is chemically known as N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl) benzylamine hydrochloride. Its molecular formula is C18H22ClNO·HCl.
The chemical structure is:
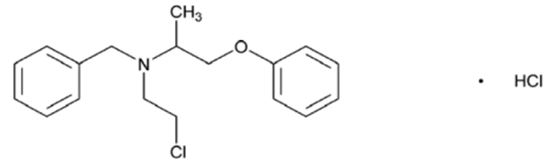
Phenoxybenzamine hydrochloride, USP is a white to almost white crystalline powder with a molecular weight of 340.29 g/mol, which melts between 136° and 141°C. It is freely soluble in ethanol (96%) and insoluble in diethyl ether.
FDA approved organic impurity specification differs from the USP.
-
CLINICAL PHARMACOLOGY
Phenoxybenzamine hydrochloride is a long-acting, adrenergic, alpha-receptor-blocking agent, which can produce and maintain “chemical sympathectomy” by oral administration. It increases blood flow to the skin, mucosa and abdominal viscera, and lowers both supine and erect blood pressures. It has no effect on the parasympathetic system.
20 percent to 30 percent of orally administered phenoxybenzamine appears to be absorbed in the active form1.
The half-life of orally administered phenoxybenzamine hydrochloride is not known; however, the half-life of intravenously administered phenoxybenzamine hydrochloride is approximately 24 hours. Demonstrable effects with intravenous administration persist for at least 3 to 4 days, and the effects of daily administration are cumulative for nearly a week1.
- INDICATION AND USAGE
- CONTRAINDICATIONS
- WARNING
-
PRECAUTIONS
General–Administer with caution in patients with marked cerebral or coronary arteriosclerosis or renal damage. Adrenergic blocking effect may aggravate symptoms of respiratory infections.
Drug Interactions2 – Phenoxybenzamine hydrochloride may interact with compounds that stimulate both alpha- and beta-adrenergic receptors (i.e. epinephrine) to produce an exaggerated hypotensive response and tachycardia (see WARNING).
Phenoxybenzamine hydrochloride blocks hyperthermia production by levarterenol, and blocks hypothermia production by reserpine.
Carcinogenesis and Mutagenesis
Case reports of carcinoma in humans after long-term treatment with phenoxybenzamine have been reported. Hence long-term use of phenoxybenzamine is not recommended3, 4. Carefully weigh the benefits and risks before prescribing phenoxybenzamine hydrochloride.
Phenoxybenzamine hydrochloride showed in vitro mutagenic activity in the Ames test and mouse lymphoma assay; it did not show mutagenic activity in vivo in the micronucleus test in mice. In rats and mice, repeated intraperitoneal administration of phenoxybenzamine hydrochloride (three times per week for up to 52 weeks) resulted in peritoneal sarcomas. Chronic oral dosing in rats (for up to 2 years) produced malignant tumors of the small intestine and non-glandular stomach, as well as ulcerative and/or erosive gastritis of the glandular stomach. Whereas squamous cell carcinomas of the non-glandular stomach were observed at all tested doses of phenoxybenzamine hydrochloride, there was a no-observed-effect-level of 10 mg/kg for tumors (carcinomas and sarcomas) of the small intestine. This dose is, on a body surface area basis, about twice the maximum recommended human dosage of 20 mg b.i.d.
Pregnancy - Teratogenic Effects
Adequate reproductive studies in animals have not been performed with phenoxybenzamine hydrochloride. It is also not known whether phenoxybenzamine hydrochloride can cause fetal harm when administered to a pregnant woman. Phenoxybenzamine hydrochloride should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether phenoxybenzamine hydrochloride is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from phenoxybenzamine hydrochloride, a decision should be made whether to discontinue nursing or to discontinue phenoxybenzamine hydrochloride, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
The following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency.
Autonomic Nervous System*: Postural hypotension, tachycardia, inhibition of ejaculation, nasal congestion, miosis.
*These so-called “side effects” are actually evidence of adrenergic blockade and vary according to the degree of blockade.
Miscellaneous: Gastrointestinal irritation, drowsiness, fatigue.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
SYMPTOMS - These are largely the result of blocking of the sympathetic nervous system and of the circulating epinephrine. They may include postural hypotension, resulting in dizziness or fainting; tachycardia, particularly postural; vomiting; lethargy; shock.
Treatment
When symptoms and signs of overdosage exist, discontinue phenoxybenzamine hydrochloride capsules. Treatment of circulatory failure, if present, is a prime consideration. In cases of mild overdosage, recumbent position with legs elevated usually restores cerebral circulation. In the more severe cases, the usual measures to combat shock should be instituted. Usual pressor agents are not effective. Epinephrine is contraindicated because it stimulates both alpha- and beta- receptors; since alpha- receptors are blocked, the net effect of epinephrine administration is vasodilation and a further drop in blood pressure (epinephrine reversal).
The patient may have to be kept flat for 24 hours or more in the case of overdose, as the effect of the drug is prolonged. Leg bandages and an abdominal binder may shorten the period of disability.
Intravenous infusion of levarterenol bitartrate** may be used to combat severe hypotensive reactions, because it stimulates alpha- receptors primarily. Although phenoxybenzamine hydrochloride is an alpha-adrenergic blocking agent, a sufficient dose of levarterenol bitartrate will overcome this effect.
The oral LD50 for phenoxybenzamine hydrochloride is approximately 2,000 mg/kg in rats and approximately 500 mg/kg in guinea pigs.
-
DOSAGE AND ADMINISTRATION
The dosage should be adjusted to fit the needs of each patient. Small initial doses should be slowly increased until the desired effect is obtained or the side effects from blockade become troublesome. After each increase, the patient should be observed on that level before instituting another increase. The dosage should be carried to a point where symptomatic relief and/or objective improvement are obtained, but not so high that the side effects from blockade become troublesome.
Initially, 10 mg of phenoxybenzamine hydrochloride twice a day. Dosage should be increased every other day, usually to 20 mg to 40 mg 2 or 3 times a day, until an optimal dosage is obtained, as judged by blood pressure control.
Long-term use of phenoxybenzamine is not recommended (see PRECAUTIONS - Carcinogenesis and Mutagenesis).
- HOW SUPPLIED
- STORAGE
-
REFERENCES
- Weiner, N.: Drugs That Inhibit Adrenergic Nerves and Block Adrenergic Receptors, in Goodman, L., and Gilman, A., The Pharmacological Basis of Therapeutics, ed. 6, New York, Macmillan Publishing Co., 1980, p. 179; p. 182.
- Martin, E.W.: Drug Interactions Index 1978/1979, Philadelphia, J.B. Lippincott Co., 1978, pp. 209-210.
- Nettesheim O, Hoffken G, Gahr M, Breidert M: Haematemesis and dysphagia in a 20-year-old woman with congenital spine malformation and situs inversus partialis [German]. Zeitschrift fur Gastroenterologie. 2003;41(4):319-24.
- Vaidyanathan S, Mansour P, Soni BM, Hughes PL, Singh G: Chronic lymphocytic leukaemia, synchronous small cell carcinoma and squamous neoplasia of the urinary bladder in a paraplegic man following long-term phenoxybenzamine therapy. Spinal Cord. 2006;44(3):188-91.
** Available as Levophed® Bitartrate (brand of norepinephrine bitartrate) from Abbott Laboratories.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Amneal Pharmaceuticals LLC.
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Oral Solid Dosage Unit
Ahmedabad 382213, INDIADistributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 12-2021-03
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHENOXYBENZAMINE HYDROCHLORIDE
phenoxybenzamine hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60219-1502 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOXYBENZAMINE HYDROCHLORIDE (UNII: X1IEG24OHL) (PHENOXYBENZAMINE - UNII:0TTZ664R7Z) PHENOXYBENZAMINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red (red cap and red body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code Amneal;1502 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60219-1502-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212568 10/30/2020 Labeler - Amneal Pharmaceuticals NY LLC (123797875) Registrant - Amneal Pharmaceuticals of New York, LLC (123797875)