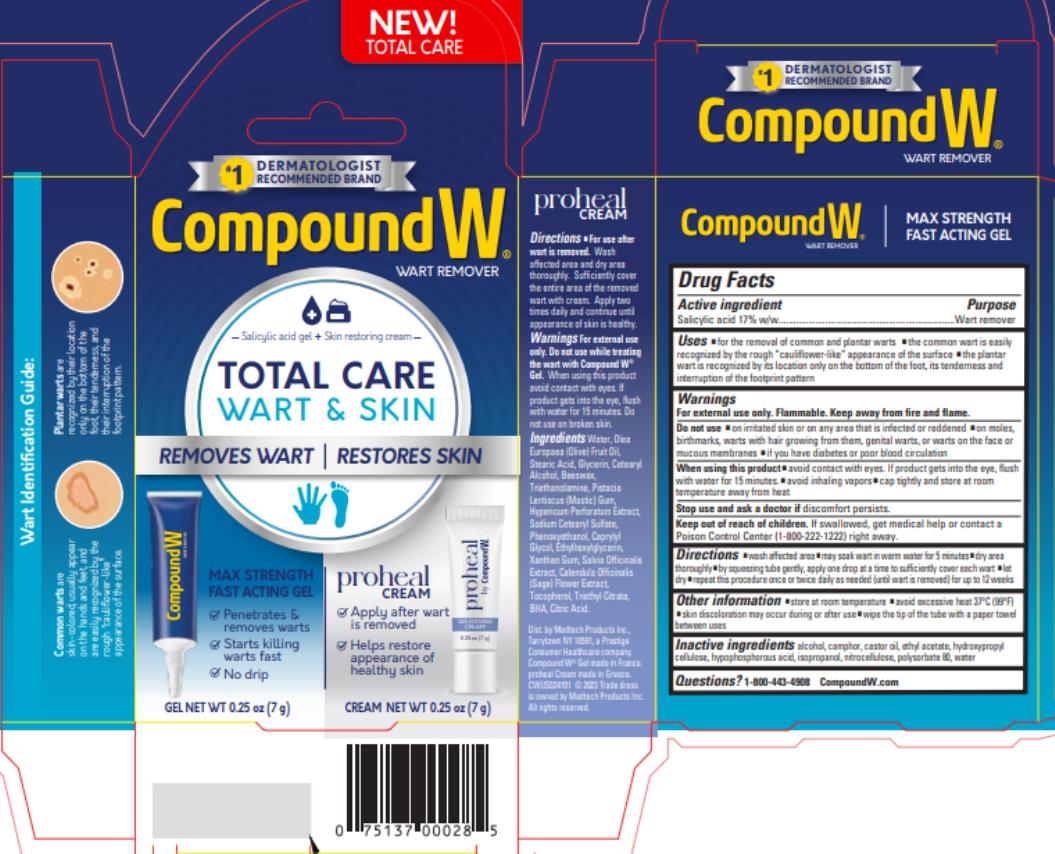
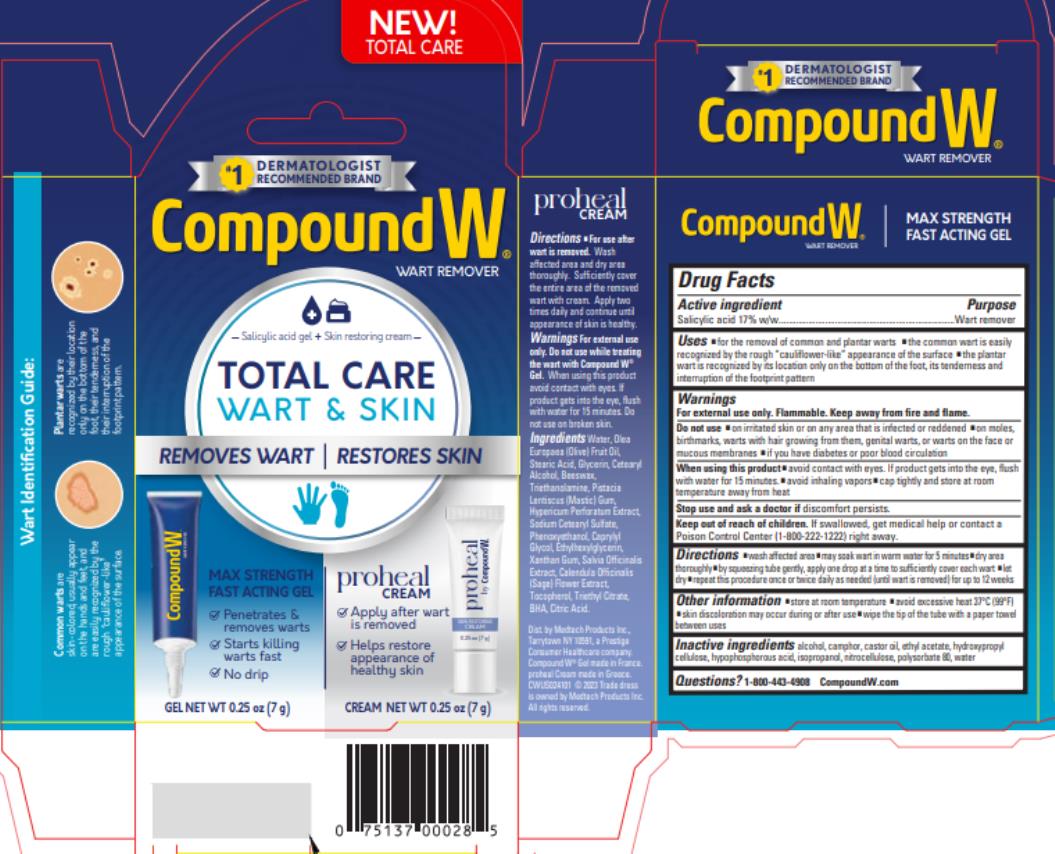
Label: COMPOUND W- salicylic acid gel
- NDC Code(s): 63029-595-12, 63029-595-14, 63029-595-25
- Packager: Medtech Products Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 2, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
Flammable. Keep away from fire and flame.
Do not use
- on irritated skin or on any area that is infected or reddened
- on moles, birthmarks, warts with hair growing from them, genital warts or warts on the face or mucous membranes
- if you have diabetes or poor blood circulation
- on irritated skin or on any area that is infected or reddened
- Directions
- Other Information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COMPOUND W
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-595 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 170 mg in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CAMPHOR (NATURAL) (UNII: N20HL7Q941) CASTOR OIL (UNII: D5340Y2I9G) ETHYL ACETATE (UNII: 76845O8NMZ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPOPHOSPHOROUS ACID (UNII: 8B1RL9B4ZJ) ISOPROPYL ALCOHOL (UNII: ND2M416302) PYROXYLIN (UNII: KYR8BR2X6O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-595-25 1 in 1 CARTON 03/15/2014 1 7 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:63029-595-12 1 in 1 CARTON 03/15/2014 2 7 g in 1 TUBE; Type 1: Convenience Kit of Co-Package 3 NDC:63029-595-14 1 in 1 CARTON 03/15/2014 3 7 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 03/15/2014 Labeler - Medtech Products Inc. (122715688)