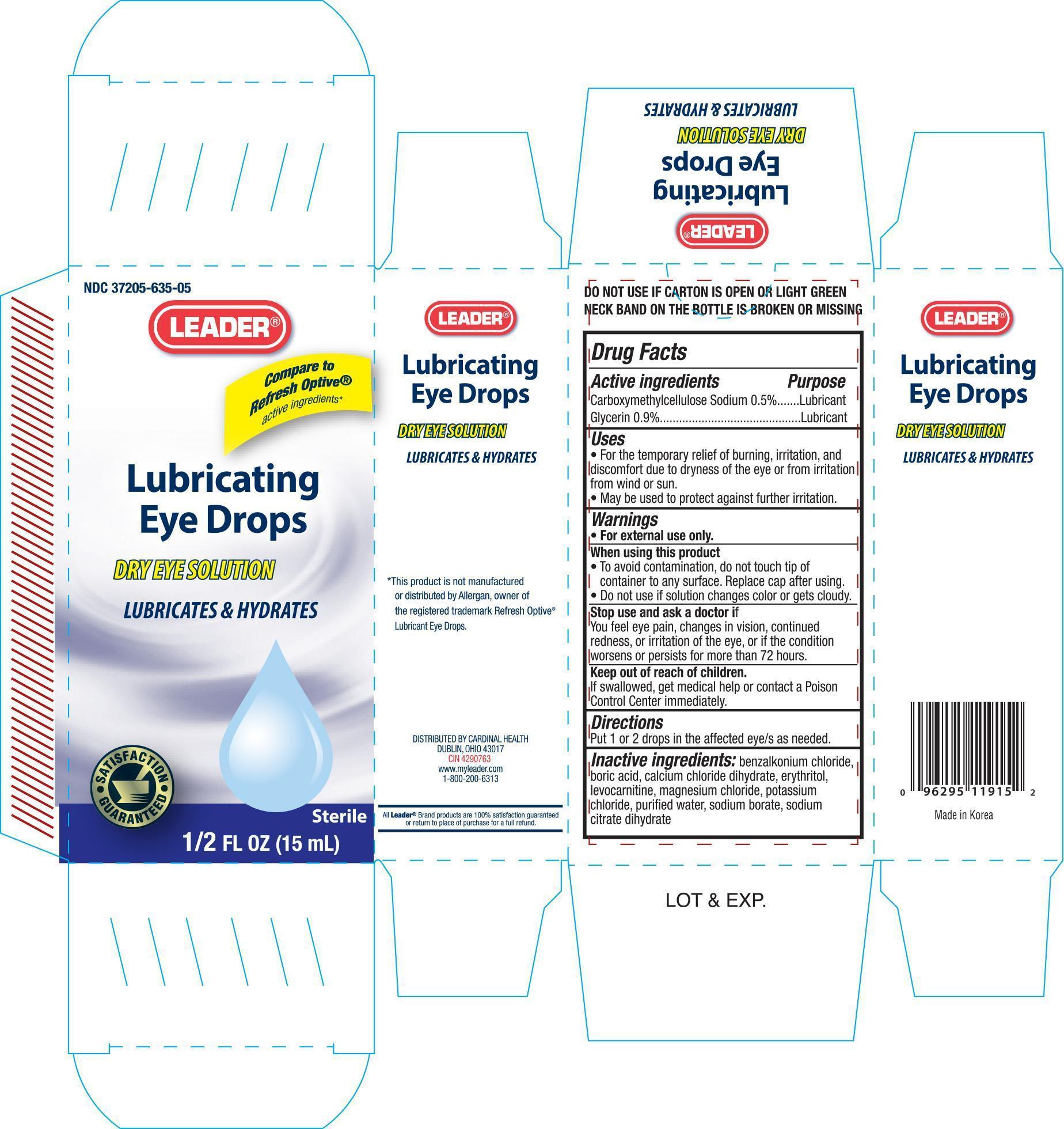
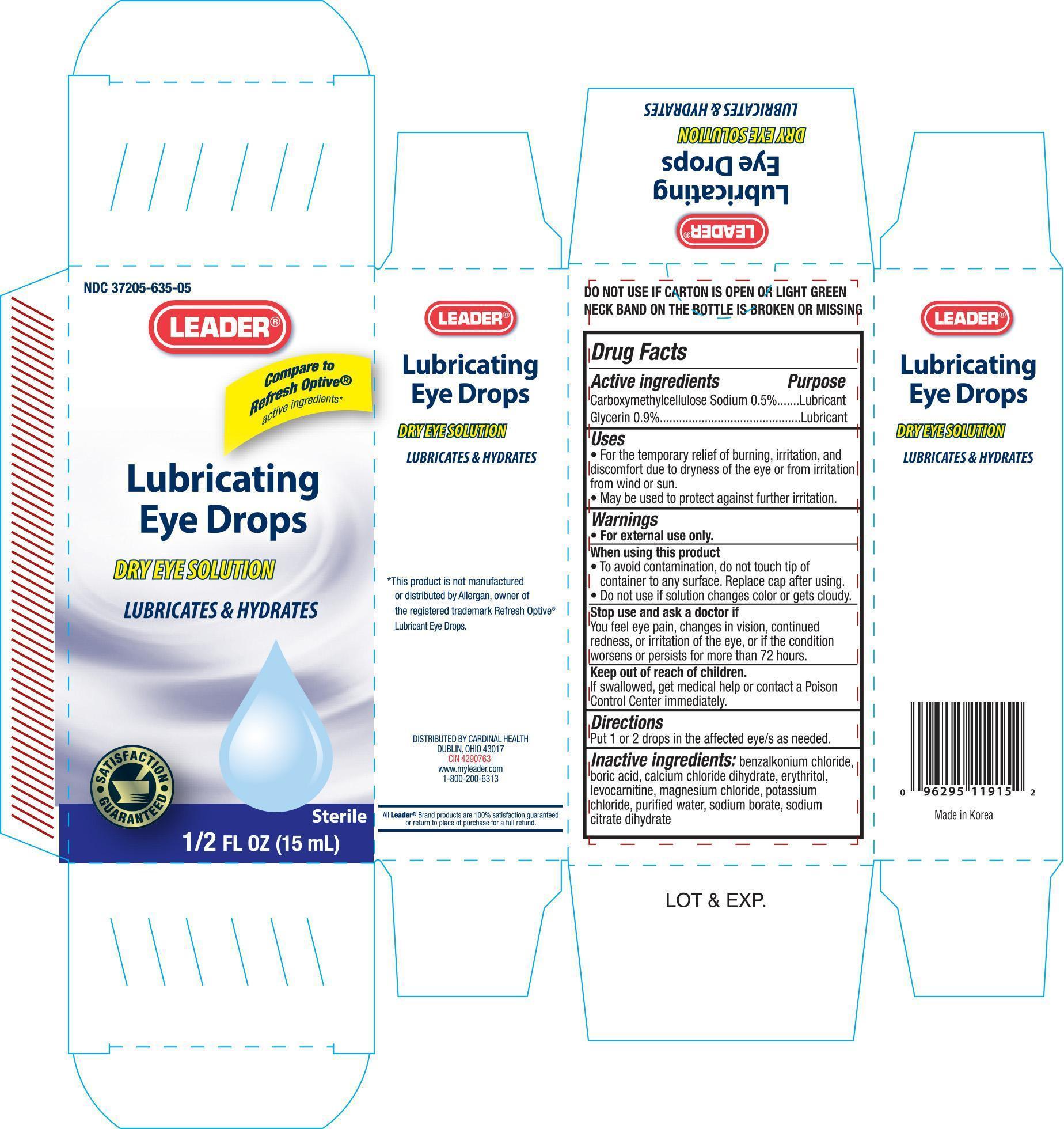
Label: LEADER LUBRICATING EYE- carboxymethylcellulose sodium, and glycerin solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 37205-635-05 - Packager: CARDINAL HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEADER LUBRICATING EYE
carboxymethylcellulose sodium, and glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37205-635 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) (CARBOXYMETHYLCELLULOSE - UNII:05JZI7B19X) CARBOXYMETHYLCELLULOSE SODIUM 5 mg in 1 mL GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) CALCIUM CHLORATE DIHYDRATE (UNII: BBB2RG413E) ERYTHRITOL (UNII: RA96B954X6) LEVOCARNITINE (UNII: 0G389FZZ9M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) WATER (UNII: 059QF0KO0R) SODIUM BORATE (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37205-635-05 1 in 1 CARTON 1 15 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 01/04/2012 Labeler - CARDINAL HEALTH (097537435)