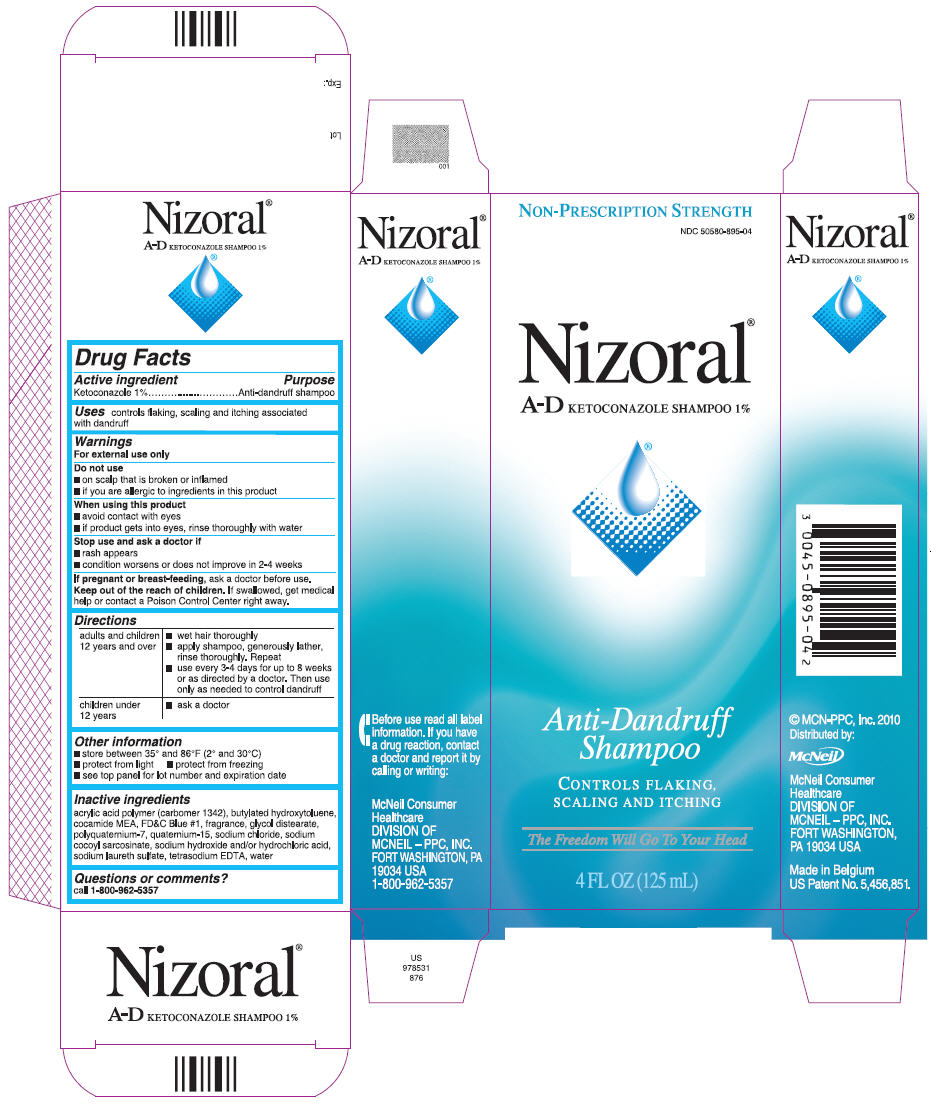
NIZORAL A-D- ketoconazole shampoo
Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division
----------
Nizoral
®
A-D KETOCONAZOLE SHAMPOO 1%
Warnings
For external use only
Directions
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
- store between 35° and 86°F (2° and 30°C)
- protect from light
- protect from freezing
- see top panel for lot number and expiration date
Inactive ingredients
acrylic acid polymer (carbomer 1342), butylated hydroxytoluene, cocamide MEA, FD&C Blue #1, fragrance, glycol distearate, polyquaternium-7, quaternium-15, sodium chloride, sodium cocoyl sarcosinate, sodium hydroxide and/or hydrochloric acid, sodium laureth sulfate, tetrasodium EDTA, water-
| NIZORAL
A-D
ketoconazole shampoo |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division (878046358) |
Revised: 9/2021
Document Id: cc90f30c-824b-5b0f-e053-2a95a90ae4da
Set id: 0ae873af-1307-4cfb-b508-988e08bd8499
Version: 5
Effective Time: 20210922
Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division