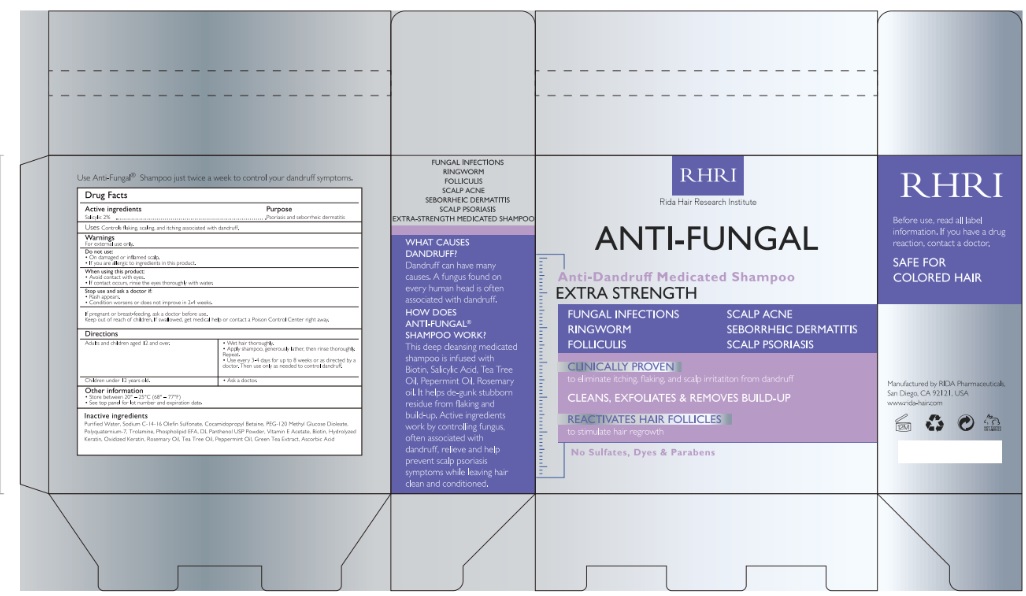
Label: RIDA HAIR RESEARCH INSTITUTE- salicylic acid liquid
- NDC Code(s): 83004-001-01
- Packager: Rida LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- On damaged or inflamed scalp
- if you are allergic to ingredients in this product
When using this product
- Avoid contact with eyes
- if contact occurs, rinse the eyes thoroughly with water
Stop use and ask a doctor if
- rash appears
- condition worsens or does not improve in 2-4 weeks.
If pregnant or breast-feeding, ask a doctor use.
Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive ingredients
- Product label
-
INGREDIENTS AND APPEARANCE
RIDA HAIR RESEARCH INSTITUTE
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83004-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) TROLAMINE (UNII: 9O3K93S3TK) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TEA TREE OIL (UNII: VIF565UC2G) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ASCORBIC ACID (UNII: PQ6CK8PD0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83004-001-01 1 in 1 CARTON 03/08/2023 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/08/2023 Labeler - Rida LLC (004425803)