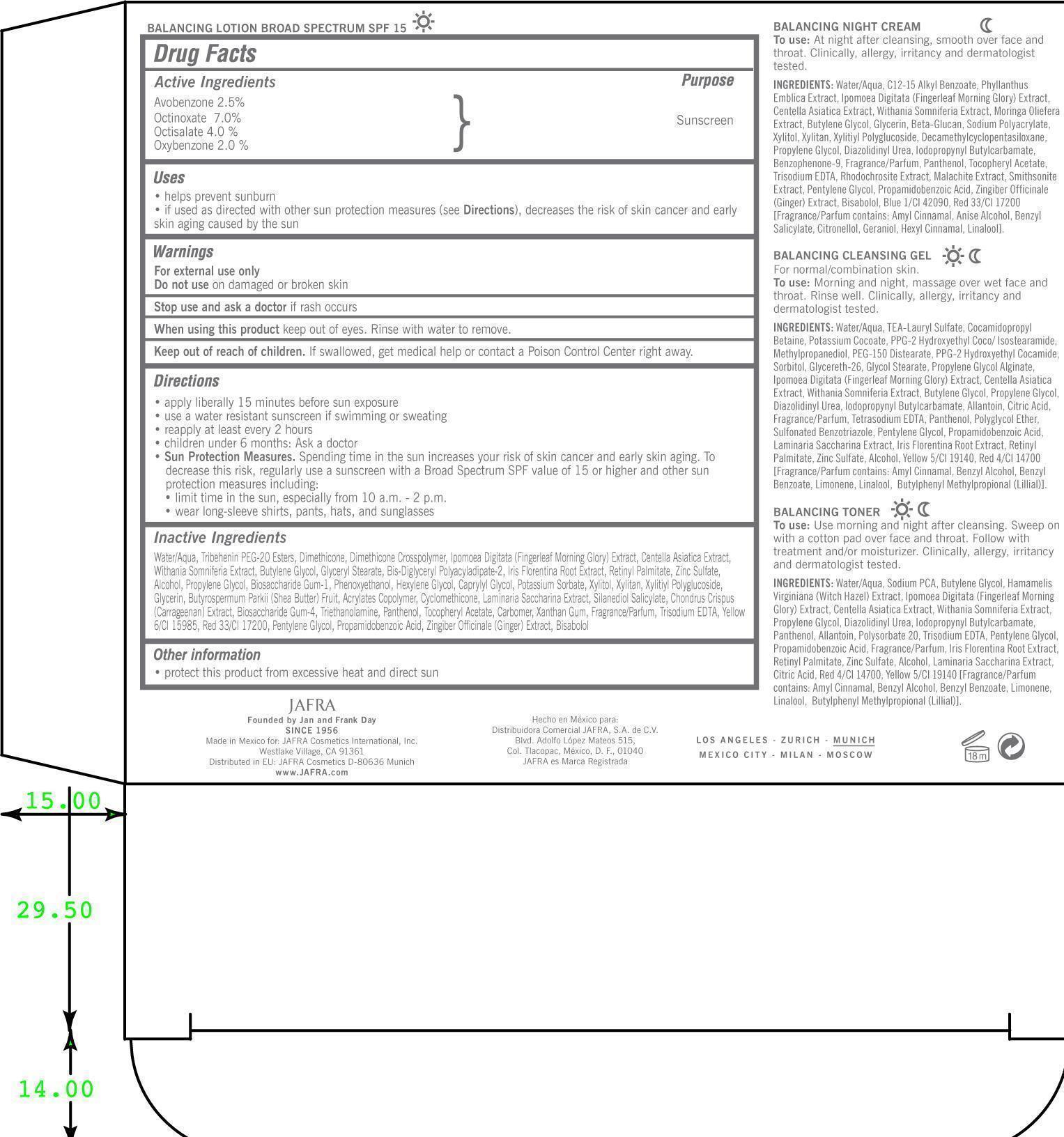
Stop use and ask a doctor if rash occurs.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen, if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: ask a doctor
Sun Protection measures.
Spending time in the sun increases risk of skin cancer and early skin aging. To decrease this risk, regulalrly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. to 2 p.m.
- Wear long sleeve shirts, pants, hats, and sunglasses
Water/Aqua, Tribehenin PEG-20 Esters, Dimethicone, Dimethicone Crosspolymer, Ipomoea Digitata (Fingerleaf Morning Glory) Extract, Centella Asiatica Extract, Withania Somniferia Extract, Butylene Glycol, Glyceryl Stearate, Bis-Diglyceryl Polyacyladipate-2, Iris Florentina Root Extract, Retinyl Palmitate, Zinc Sulfate, Alcohol, Propylene Glycol, Biosaccharide Gum-1, Phenoxyethanol, Hexylene Glycol, Caprylyl Glycol, Potassium Sorbate, Xylitol, Xylitan, Xylitiyl Polyglucoside, Glycerin, Butyrospermum Parkii (Shea Butter) Fruit, Acrylates Copolymer, Cyclomethicone, Laminaria Saccharina Extract, Silanediol Salicylate, Chondrus Crispus (Carrageenan) Extract, Biosaccharide Gum-4, Triethanolamine, Panthenol, Tocopheryl Acetate, Carbomer, Xanthan Gum, Fragrance/Parfum, Trisodium EDTA, Yellow 6/CI 15985, Red 33/CI 17200, Pentylene Glycol, Propamidobenzoic Acid, Zingiber Officinale (Ginger) Extract, Bisabolol.