Label: QWO- collagenase clostridium histolyticum-aaes injection, powder, lyophilized, for solution
- NDC Code(s): 73611-300-05, 73611-300-10
- Packager: Endo Aesthetics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QWO safely and effectively. See full prescribing information for QWO.
QWO® (collagenase clostridium histolyticum-aaes) for injection, for subcutaneous use
Initial U.S. Approval: 2020
INDICATIONS AND USAGE
QWO is a combination of bacterial collagenases indicated for the treatment of moderate to severe cellulite in the buttocks of adult women. (1)
DOSAGE AND ADMINISTRATION
- A treatment area is defined as a single buttock receiving up to 12 injections, 0.3 mL each (up to a total of 3.6 mL), of QWO. (2.1)
- A treatment visit may consist of up to 2 treatment areas. Treatment visits should be repeated every 21 days for 3 treatment visits. (2.1)
- Reconstitute QWO lyophilized powder with the supplied diluent prior to use. (2.2)
- Inject 0.84 mg of QWO per treatment area as 12 subcutaneous injections (0.3-mL injection administered as three 0.1-mL aliquots per injection). (2.3)
DOSAGE FORMS AND STRENGTHS
For injection: 0.92 mg or 1.84 mg as a lyophilized powder in single-dose vials. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis may occur with collagenase clostridium histolyticum. If a serious hypersensitivity reaction occurs, initiate appropriate therapy. (5.1)
- Injection Site Bruising: Bruising occurs frequently after QWO administration. Use with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet (except those taking ≤ 150 mg aspirin daily) or anticoagulant therapy. (5.2)
- Substitution: QWO must not be substituted for other injectable collagenase products. QWO is not indicated for the treatment of Peyronie’s disease or Dupuytren’s contracture. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) were related to the injection site (bruising, pain, nodule, pruritus, erythema, discoloration, swelling, and warmth). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Endo Pharmaceuticals Inc. at 1-800-462-3636 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Reconstitution of Lyophilized Powder
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Injection Site Bruising
5.3 Substitution of Collagenase Products
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
QWO is injected subcutaneously at a dose of 0.84 mg per treatment area.
- A treatment area is defined as a single buttock receiving up to 12 injections, 0.3 mL each (up to a total of 3.6 mL), of QWO.
- A treatment visit may consist of up to 2 treatment areas.
Treatment should be repeated every 21 days for 3 treatment visits.
2.2 Reconstitution of Lyophilized Powder
Before reconstitution, remove the vials from the refrigerator and let stand at room temperature for at least 15 minutes. Inspect the vials containing QWO. The cake of lyophilized powder should be white in color and intact, showing no signs of erosion. The diluent should be a colorless solution, free of particulate matter.
After removal of the flip-off cap from the vial(s), using aseptic technique swab the rubber stopper and surrounding surface of the vial(s) containing QWO and diluent with sterile alcohol (no other antiseptics should be used).
Use only the supplied diluent for the reconstitution of QWO.
Using an appropriate sized syringe and needle (not supplied), withdraw the amount of supplied diluent based on the number of injection sites (see Table 1).
Table 1: Reconstitution Instructions for QWO Single Treatment Area Two Treatment Areas Collagenase clostridium histolyticum-aaes (mg) 0.92 1.84 Volume of diluent (mL) 4 8 Concentration after reconstitution (mg/mL) 0.23 0.23 Number of treatment areas 1 2 Inject the diluent slowly into the sides of the vial containing the lyophilized powder of QWO. Do not invert the vial or shake the solution. Slowly swirl the solution to ensure that all of the lyophilized powder has gone into solution.
The reconstituted QWO solution in the vial can be kept at room temperature (20°C to 25°C/68°F to 77°F) for up to 8 hours or refrigerated at 2°C to 8°C (36°F to 46°F) for up to 72 hours prior to administration. If the reconstituted QWO solution in the vial is refrigerated, allow this solution to return to room temperature for approximately 15 minutes before use.
The reconstituted QWO solution should be clear, colorless and free of particulate matter. Inspect the solution visually for particulate matter or discoloration prior to administration. If the reconstituted QWO is not a clear, colorless solution essentially free of particulate matter, do not inject it.
Discard the syringe(s) and needle(s) used for reconstitution and the diluent vial(s).
After reconstitution, QWO solution in the vial should be used for only one injection session and for only one patient.
2.3 Administration
Preparation of Syringes for Injection
Using 1-mL syringes with removable needles (not supplied), draw up 0.9 mL of the reconstituted solution into each syringe. See Table 2 for the appropriate number of syringes needed based on the number of injection sites. After the syringes are prepared, pull the solution remaining in the needles into the barrels of the syringe and then replace the needle with a 30-gauge 1/2-inch needle. Administer reconstituted solution prepared in 1-mL syringes immediately. Do not store reconstituted solution in the 1-mL syringes.
Table 2: Preparation Instructions for QWO Single Treatment Visit Two Treatment Visit Number of 1-mL syringes 4 8 Volume per syringe (mL) 0.9 0.9 Amount of collagenase clostridium histolyticum-aaes per syringe (mg) 0.21 0.21 Total injection volume (mL) in prepared syringes 3.6 7.2 Total injection amount of collagenase clostridium histolyticum-aaes (mg) in prepared syringes 0.84 1.68 Injection Technique
Mark the injection sites while the patient is standing. Inject QWO subcutaneously while the patient is in a prone position. Each injection of QWO should be administered as three 0.1-mL aliquots to positions A, B, and C (for a total injection volume of 0.3 mL) as shown in the following figure. The depth of the injection should be 0.5 inches (corresponding to the length of the needle) without downward pressure.
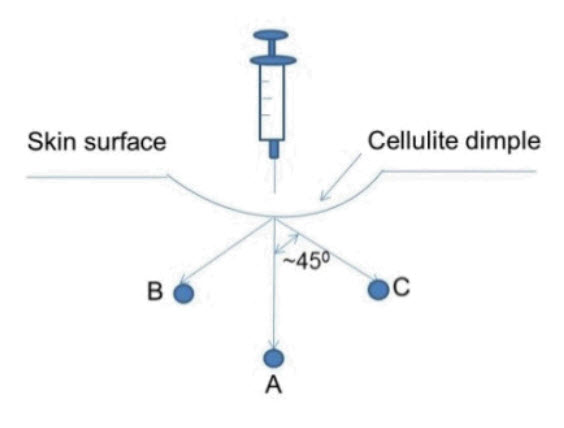
Needle Tip Position A: Position the needle at 90° angle perpendicular to the skin surface at the injection site and inject one 0.1-mL aliquot by gently pushing on the syringe plunger.
Needle Tip Position B: Withdraw the needle slightly (but not so much as to remove from the injection site) and reposition approximately 45° (but not more than 45°) and inject one 0.1-mL aliquot (towards head).
Needle Tip Position C: Withdraw the needle slightly (but not so much as to remove from the injection site) and reposition approximately 45° (but not more than 45°) and inject one 0.1-mL aliquot, (towards foot).
Withdraw needle from the skin completely and move to the next identified injection site. Each treatment area may receive up 12 injections. After treatment the patient should remain prone for at least 5 minutes.
Do not store, pool, or use any vials or syringes containing unused reconstituted solution after administration. Discard any unused portions.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
QWO is contraindicated in:
- patients with a history of hypersensitivity to collagenase or to any of the excipients [see Warnings and Precautions (5.1)].
- the presence of infection at the injection sites.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions including anaphylaxis have been reported with the use of collagenase clostridium histolyticum. If such a reaction occurs, further injection of QWO should be discontinued and appropriate medical therapy immediately instituted.
5.2 Injection Site Bruising
In clinical trials, 84% of subjects treated with QWO experienced injection site bruising [see Adverse Reactions (6.1)]. Subjects with coagulation disorders or using anticoagulant or antiplatelet medications (except those taking ≤ 150 mg aspirin daily) were excluded from participating in Trials 1 and 2.
QWO should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet (except those taking ≤ 150 mg aspirin daily) or anticoagulant therapy.
-
6 ADVERSE REACTIONS
The following adverse reactions to QWO for injection are discussed in greater detail in other sections of the labeling:
- Hypersensitivity [see Contraindications (4) and Warnings and Precautions (5.1)].
- Injection Site Bruising [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In two double-blind, placebo-controlled clinical trials (Trials 1 and 2) of identical design, 424 female subjects with cellulite in the buttocks received QWO and 419 female subjects with cellulite received placebo. Enrolled subjects were adults age 18 to 78 years with moderate to severe cellulite (graded as 3 or 4 on a 0 to 4 scale) and without excessive skin laxity. The majority were White (78%) or African American (18%). Subjects completed up to 3 treatment visits separated by 21 days and were followed for up to 6 months after the last treatment visit in a separate open-label extension trial (Trial 3).
Table 3 shows the incidence of adverse reactions that were reported in ≥ 1% of subjects who received QWO-and at a frequency greater than subjects who received placebo in Trials 1 and 2 through Day 71. Generally, adverse reactions had a duration of less than 21 days.
Table 3: Adverse Reactions Occurring in ≥ 1% of Subjects in Trials 1 and 2 Through Day 71 Adverse Reactions at Injection Site QWO
N=424
%Placebo
N=419
%Bruising 84 21 Pain 48 10 Nodule 33 1 Pruritus 15 1 Erythema 9 5 Discoloration 8 1 Swelling
Warmth
8
3
1
0
Pooled terms:
- Bruising - injection site bruising, injection site hematoma, and injection site hemorrhage (refers to verbatim term injection site ecchymosis)
- Pain - injection site pain, injection site discomfort, and injection site dysesthesia
- Swelling - injection site swelling, injection site edema, injection site induration
- Discoloration - injection site discoloration
- Nodule- injection site mass and injection site nodule
Four hundred seventy-nine (479) subjects from Trials 1 and 2 completed a 6-month observation phase in the ongoing open-label safety extension (Trial 3). No long-term safety signals have been identified.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handing, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products, including other collagenase clostridium histolyticum products, may be misleading.
By Day 22, approximately 53% (203/383) and 26% (101/383) of subjects who completed the first treatment visit of QWO at the recommended dose in Trials 1 and 2 developed anti-AUX-I and anti-AUX-II antibodies, respectively. The majority (> 96%) of subjects developed antibodies for AUX-I and AUX-II after second and third treatment visits. Antibody titers suggested that antibodies were retained for up to 360 days after receiving the first recommended dose. By Day 71, approximately 68% and 83% of subjects developed antibodies to AUX-I and AUX-II which were classified as neutralizing, respectively.
Antibodies to AUX-I and AUX-II including those classified as neutralizing were not associated with changes in clinical response or adverse reactions at injection site.
6.3 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reaction was reported during post approval use of a collagenase product:
Immune system disorders: serious hypersensitivity reactions including anaphylaxis[see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on collagenase clostridium histolyticum use in pregnant women to evaluate for a drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Following subcutaneous injection, the systemic concentrations for QWO were below the bioanalytical assay limit of quantification [see Clinical Pharmacology (12.3)].In animal reproduction studies, intravenous administration of collagenase clostridium histolyticum to pregnant rats during organogenesis at doses up to 0.13 mg/rat (43 × human equivalent dose [HED] on a mg/kg basis) revealed no evidence of harm to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of collagenase clostridium histolyticum in human milk, the effects of collagenase clostridium histolyticum on the breastfed child or on milk production. Following subcutaneous injection, the systemic concentrations for QWO were below the bioanalytical assay limit of quantification [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for collagenase clostridium histolyticum and any potential adverse effects on the breastfed child from collagenase clostridium histolyticum, or from the underlying maternal condition.
-
11 DESCRIPTION
Collagenase clostridium histolyticum-aaes is a combination of bacterial collagenases AUX-I and AUX-II, in an approximate 1:1 mass ratio, which are isolated and purified from the fermentation of Clostridium histolyticum bacteria.
Collagenase AUX-I is a single polypeptide chain consisting of approximately 1000 amino acids. It has an observed molecular weight of 114 kiloDaltons (kDa). It belongs to the class I Clostridium histolyticum collagenases.
Collagenase AUX-II is a single polypeptide chain consisting of approximately 1000 amino acids. It has an observed molecular weight of 113 kDa. It belongs to the class II Clostridium histolyticum collagenases.
QWO (collagenase clostridium histolyticum-aaes) for injection is supplied as a sterile, preservative-free, lyophilized powder (appearing as a white cake) in single-dose vials for subcutaneous use after reconstitution with the Diluent for QWO.
Each QWO 0.92-mg single-dose vial contains 0.92 mg of collagenase clostridium histolyticum-aaes and mannitol (37.7 mg), sucrose (18.9 mg), tromethamine (1.1 mg), and hydrochloric acid as needed to adjust pH. Reconstitution with 4 mL of supplied Diluent for QWO yields a solution containing 0.23 mg/mL collagenase clostridium histolyticum-aaes at a pH of approximately 8.0.
Each QWO 1.84-mg single-dose vial contains 1.84 mg of collagenase clostridium histolyticum-aaes and mannitol (75.4 mg), sucrose (37.8 mg), tromethamine (2.2 mg), and hydrochloric acid as needed to adjust pH. Reconstitution with 8 mL of supplied Diluent for QWO yields a solution containing 0.23 mg/mL collagenase clostridium histolyticum-aaes at a pH of approximately 8.0.
Diluent for QWO is a sterile, preservative-free, colorless solution in a single-dose vial containing either 4 mL or 8 mL of 0.03% calcium chloride dihydrate in 0.6% sodium chloride, and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Collagenases are proteinases that hydrolyze collagen in its native triple helical conformation under physiological conditions. The exact mechanism for the treatment of moderate to severe cellulite is unknown.
12.3 Pharmacokinetics
Pharmacokinetics of collagenases clostridium histolyticum were evaluated in 140 female subjects with cellulite in four clinical trials. Plasma concentrations of clostridium type I collagenase (AUX-I) and clostridium type II collagenase (AUX-II) were below the lower limit of quantitation of 5 ng/mL and 25 ng/mL, respectively, in all subjects that received a single dose of (QWO) up to 3.36 mg in up to 4 treatment areas (up to 0.84 mg per treatment area).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of collagenase clostridium histolyticum have not been conducted.
Purified collagenase clostridium histolyticum was not mutagenic in Salmonella typhimurium (Ames test) and was not clastogenic in both an in vivo mouse micronucleus assay and an in vitro chromosomal aberration assay in human lymphocytes.
Collagenase clostridium histolyticum did not impair fertility and early embryonic development when administered intravenously to rats at doses up to 0.13 mg/rat (43 × HED on a mg/kg basis).
-
14 CLINICAL STUDIES
Two randomized, multicenter, double-blind, placebo-controlled trials, Trial 1 and Trial 2, of identical design were conducted to evaluate safety and efficacy of QWO for the treatment of cellulite in adult women. Eligible subjects had cellulite severity in both buttocks of moderate (3) to severe (4) as evaluated on 5-level scales (0=none; 4=severe) by both the subject, using the Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS), and the investigator, using the Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS).
A dose of 0.84 mg of QWO per buttock was administered as 12 subcutaneous injections (0.3-mL injection administered as three 0.1-mL aliquots per injection) in each of 2 buttocks for a total dose of 1.68 mg and a total volume of 7.2 mL (3.6 mL per buttock) per treatment visit. There were 3 treatment visits at 21-day intervals.
In Trials 1 and 2, the primary efficacy endpoint was the proportion of 2-level multi-component responders at Day 71 post randomization. A 2-level multi-component responder was defined as having an improvement of at least 2 levels of cellulite severity from baseline on both the CR-PCSS and the PR-PCSS in the target buttock.
Patient satisfaction with the appearance of their cellulite was assessed using a patient reported outcome scale ranging from 0 (extremely dissatisfied) to 6 (extremely satisfied).
The mean age was 47 years with a mean BMI of 31 kg/m2. All of the subjects were female, and most were White (78%). At baseline, 61% subjects had investigator reported cellulite severity (CR-PCSS) scores of moderate and 39% subjects had cellulite severity scores of severe.
Reductions in cellulite severity were observed more frequently in the QWO group compared to the placebo group as measured by the investigator (CR-PCSS) and patient (PR-PCSS) scales at Day 71 (Table 4).
Table 4: Subject/Investigator 2-Level Responder Analysis at Day 71 Trial 1 Trial 2 QWO
N=210Placebo
N=213Adj Trt Diff
(95% CI)QWO
N=214Placebo
N=206Adj Trt Diff
(95% CI)2-level multi-component responder 16 (8%) 4 (2%) 6%
(2%, 10%)12 (6%) 1 (< 1%) 5%
(2%, 8%)2-level PR‑PCSS responder 51 (24%) 26 (12%) 12%
(5%, 19%)45 (21%) 12 (6%) 15%
(9%, 22%)2-level CR‑PCSS responder 35 (17%) 12 (6%) 11%
(5%, 17%)32 (15%) 3 (1%) 13%
(8%, 19%)Adj Trt Diff=Adjusted treatment difference; CI=Confidence interval
Non-responder imputation used to handle missing data. Adjusted treatment difference is the weighted average of the treatment differences in response percentages across the analysis centers using Cochran-Mantel-Haenszel (CMH) weights along with the associated CI.
In Trials 1 and 2, the measure of patient-reported satisfaction with cellulite appearance showed a greater improvement in the QWO group over the placebo group.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
QWO (collagenase clostridium histolyticum-aaes) for injection is a sterile, preservative-free, lyophilized powder (appearing as a white cake) in single-dose vials for subcutaneous use.
NDC Number Package Size 73611-300-05 Single treatment area:
Carton containing one QWO 0.92-mg single-dose vial and one Diluent for QWO 4-mL single-dose vial [see Description (11)]73611-300-10 Two treatment areas:
Carton containing one QWO 1.84-mg single-dose vial and one Diluent for QWO 8-mL single-dose vial [see Description (11)]Storage and Handling
Store QWO and Diluent for QWO vials at 2°C to 25°C (36°F to 77°F). Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.1)].
Injection Site Bruising
Advise patients that injection site bruising may occur with administration of QWO [see Warnings and Precautions (5.2)].
Manufactured by:
Endo Global Aesthetics Limited
Dublin, Ireland
US License No. 2136Distributed by:
Endo Aesthetics LLC
Malvern, PA 19355
-
PATIENT PACKAGE INSERT
Patient Information
QWO (kwoe)
(collagenase clostridium histolyticum-aaes)
for injection, for subcutaneous useWhat is QWO?
QWO is a prescription medicine used for the treatment of moderate to severe cellulite in the buttocks of adult women.
It is not known if QWO is safe and effective in children.
Do not receive QWO if you:
- Are allergic to any collagenase or to any of the ingredients in QWO. See the end of this Patient Information for a complete list of ingredients in QWO.
- Have an active infection in the treatment area.
Before receiving QWO, tell your healthcare provider about all of your medical conditions, including if you:
- have had an allergic reaction to a QWO injection in the past
- have a bleeding problem
- are pregnant or plan to become pregnant. It is not known if QWO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if QWO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive QWO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take a medicine that prevents the clotting of your blood (antiplatelet or anticoagulant medicine).
How will I receive QWO?
- QWO is injected into the fat (subcutaneously) of each single buttock (treatment area) by your healthcare provider. You may receive up to 12 injections per treatment area.
- Each treatment visit may include up to 2 treatment areas.
- QWO injections will be given 21 days apart for 3 treatment visits.
What are the possible side effects of QWO?
QWO may cause serious side effects, including:
-
Allergic (hypersensitivity) reactions, including anaphylaxis. Call your healthcare provider right away if you have any of these symptoms of an allergic reaction after an injection of QWO:
- hives
- swollen face
- trouble breathing
- chest pain
- low blood pressure
- dizziness or fainting
- Injection site bruising.
The most common side effects of QWO include bruising, pain, areas of hardness, itching, redness, discoloration, swelling and warmth in the treatment area.
These are not all of the possible side effects of QWO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about QWO
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for more information about QWO that is written for health professionals.
What are the ingredients in QWO?
Active ingredient: collagenase clostridium histolyticum
Inactive ingredients: mannitol, sucrose, tromethamine, and hydrochloric acid
The diluent contains: calcium chloride dihydrate, sodium chloride, and Water for Injection, USP
Manufactured by: Endo Global Aesthetics Limited, Dublin, Ireland US license number 2136 Distributed by: Endo Aesthetics LLC , Malvern, PA 19355
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 07/2020
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QWO
collagenase clostridium histolyticum-aaes injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73611-300 Route of Administration INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLLAGENASE CLOSTRIDIUM HISTOLYTICUM (UNII: 9X7O8V25IT) (COLLAGENASE CLOSTRIDIUM HISTOLYTICUM - UNII:9X7O8V25IT) COLLAGENASE CLOSTRIDIUM HISTOLYTICUM 0.23 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73611-300-05 1 in 1 CARTON 02/01/2021 01/31/2025 1 4 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:73611-300-10 1 in 1 CARTON 02/01/2021 01/31/2025 2 8 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761146 02/01/2021 01/31/2025 Labeler - Endo Aesthetics LLC (117361103) Establishment Name Address ID/FEI Business Operations Auxilium Pharmaceuticals, LLC 801535787 api manufacture(73611-300) Establishment Name Address ID/FEI Business Operations Par Sterile Products LLC 808402890 manufacture(73611-300) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 pack(73611-300)


