Label: IBUPROFEN suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 76413-312-15 - Packager: Central Texas Community Health Centers
- This is a repackaged label.
- Source NDC Code(s): 49348-374
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 25, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 1.25 mL)
- Purposes
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- •
- hives
- •
- facial swelling
- •
- asthma (wheezing)
- •
- shock
- •
- skin reddening
- •
- rash
- •
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding.
The chance is higher if your child:
- •
- has had stomach ulcers or bleeding problems
- •
- takes a blood thinning (anticoagulant) or steroid drug
- •
- takes other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- •
- takes more or for a longer time than directed
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
Do not use
- •
- if the child has ever had an allergic reaction to any other pain reliever/fever reducer
- •
- right before or after heart surgery
Ask a doctor before use if
- •
- stomach bleeding warning applies to your child
- •
- child has a history of stomach problems, such as heartburn
- •
- child has problems or serious side effects from taking pain relievers or fever reducers
- •
- child has not been drinking fluids
- •
- child has lost a lot of fluid due to vomiting or diarrhea
- •
- child has high blood pressure, heart disease, liver cirrhosis, or kidney disease
- •
- child has asthma
- •
- child is taking a diuretic
Ask a doctor or pharmacist before use if the child is
- •
- under a doctor's care for any serious condition
- •
- taking any other drug
When using this product
- •
- give with food or milk if stomach upset occurs
- •
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- •
- child experiences any of the following signs of stomach bleeding:
- •
- feels faint
- •
- vomits blood
- •
- has bloody or black stools
- •
- has stomach pain that does not get better
- •
- the child does not get any relief within first day (24 hours) of treatment
- •
- fever or pain gets worse or lasts more than 3 days
- •
- redness or swelling is present in the painful area
- •
- any new symptoms appear
-
Directions
- •
- this product does not contain directions or complete warnings for adult use
- •
- do not give more than directed
- •
- shake well before using
- •
- find right dose on chart below. If possible, use weight to dose; otherwise use age.
- •
- measure with the dosing device provided. Do not use with any other device.
- •
- dispense liquid slowly into the child's mouth, toward the inner cheek
- •
- if needed, repeat dose every 6-8 hours
- •
- do not use more than 4 times a day
Dosing Chart
Weight (lbs)
Age (mos)
Dose (mL)
under 6 mos
ask a doctor
12-17 lbs
6-11 mos
1.25 mL
18-23 lbs
12-23 mos
1.875 mL
- Other information
- Inactive ingredients
-
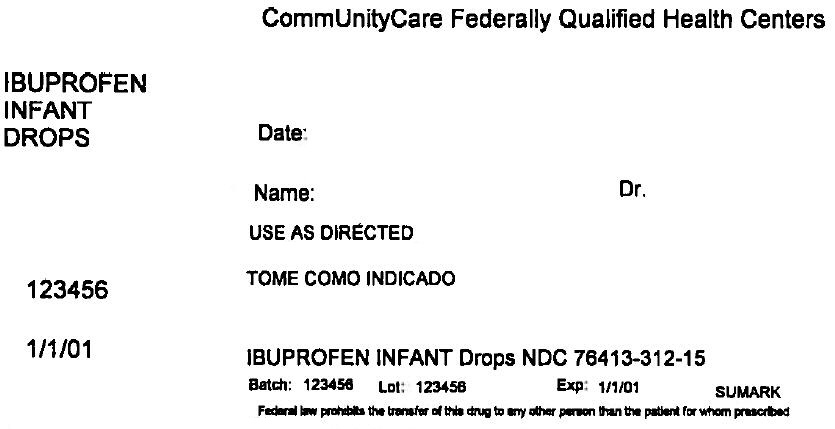
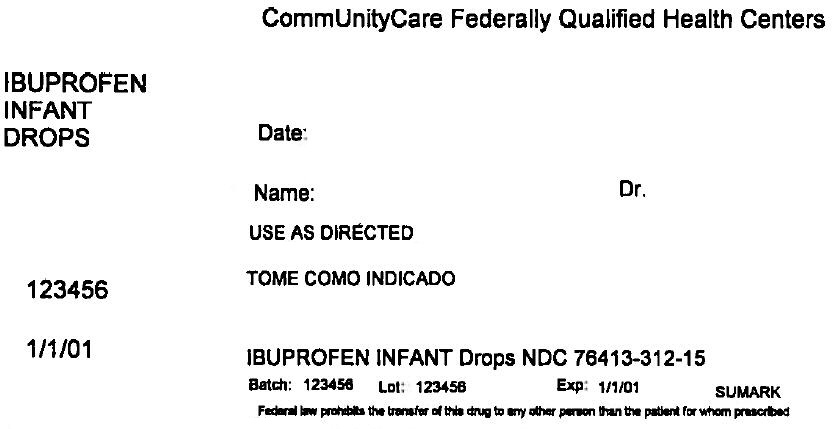
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Label
CommUnityCare Federally Qualified Health Centers
IBUPROFEN
INFANT
DROPSDate:
Name:
Dr.USE AS DIRECTED
123456
1/1/01
IBUPROFEN INFANT Drops NDC 76413-312-15
Batch: 123456
Lot: 123456
Exp: 1/1/01SUMARK
Federal law prohibits the transfer of this drug to any other person than the patient for whom prescribed
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76413-312(NDC:49348-374) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 50 mg in 1.25 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Xanthan Gum (UNII: TTV12P4NEE) Product Characteristics Color PINK (light) Score Shape Size Flavor FRUIT (mixed) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76413-312-15 1 in 1 CARTON 08/28/2003 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075217 08/28/2003 Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Central Texas Community Health Centers 079674019 REPACK(76413-312) , RELABEL(76413-312)