Label: INATAL ADVANCE tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 63044-153-01, 63044-153-64 - Packager: Nnodum Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 26, 2009
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Prenatal multivitamin/multimineral tablet
combining 15 vitamins and minerals,
including calcium and carbonyl iron.Rx Only
WARNING: Accidental overdose of iron containing prod-ucts is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison controll center immediately. -
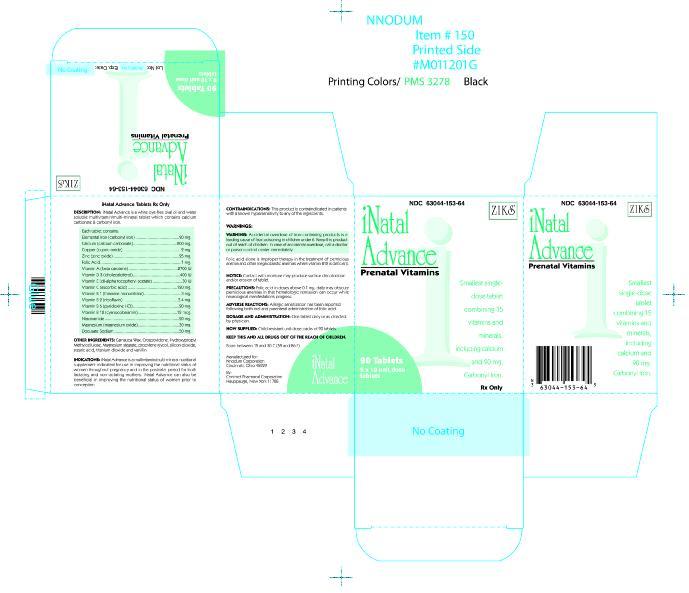
DESCRIPTION
INATAL Advanced ® is a white, dye-free, oval shaped, oil-and-water soluble multivitamin/multimineral tablet which contains calcium carbonate and carbonyl iron.
Each tablet contains:
Vitamin A (as beta-carotene) . . . . . . . . . . . 2700 I.U.
Vitamin C (ascorbic acid) . . . . . . . . . . . . . . 120 mg
Calcium (as calcium carbonate) . . . . . . . . . . 200 mg
Elemental Iron (as carbonyl iron) . . . . . . . . . . 90 mg
Vitamin D3 (cholecalciferol) . . . . . . . . . . . . 400 I.U.
Vitamin E (dl-alpha tocopheryl acetate) . . . . . 30 I.U.
Vitamin B1 (as thiamine mononitrate) . . . . . . . . 3 mg
Vitamin B2 (riboflavin, USP) . . . . . . . . . . . . . 3.4 mg
Niacinamide . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Vitamin B6 (as pyridoxine HCI, USP) . . . . . . . 20 mg
Folic Acid, USP . . . . . . . . . . . . . . . . . . . . . . . 1 mg
Vitamin B12 (cyanocobalamin) . . . . . . . . . . . 12 mcg
Zinc (as zinc oxide, USP) . . . . . . . . . . . . . . . . 25 mg
Copper (as cupric oxide) . . . . . . . . . . . . . . . . . 2 mg
Magnesium (as magnesium oxide, USP) . . . . . 30 mg
Docusate Sodium . . . . . . . . . . . . . . . . . . . . 50 mg
INACTIVE INGREDIENTS: Carnauba wax, crospovidone, ethyl vanillin, hydroxypropyl methylcellulose, magnesium stearate, polyethylene glycol, silicon dioxide, stearic acid, and titanium dioxide
-
INDICATIONS:
INATAL ADVANCE ® is a multivitamin/multimineral nutritional supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. INATAL Advanced® can also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
Folic acid in doses above 0.1mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
GERIATRIC USE:
Clinical studies on this product have not been performed in sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually staring at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INATAL ADVANCE
inatal advance tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63044-153 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 2700 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM - UNII:SY7Q814VUP) CALCIUM CARBONATE 200 mg IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (IRON - UNII:E1UOL152H7) IRON PENTACARBONYL 90 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (ALPHA-TOCOPHEROL - UNII:H4N855PNZ1) ALPHA-TOCOPHEROL ACETATE 30 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE - UNII:X66NSO3N35) THIAMINE MONONITRATE 3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 20 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC - UNII:J41CSQ7QDS) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (COPPER - UNII:789U1901C5) CUPRIC OXIDE 2 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM OXIDE 30 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSPOVIDONE (UNII: 68401960MK) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) MAGNESIUM STEARATE (UNII: 70097M6I30) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYL VANILLIN (UNII: YC9ST449YJ) Product Characteristics Color WHITE (dye-free) Score 2 pieces Shape OVAL Size 5mm Flavor Imprint Code cpc2859 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63044-153-64 9 in 1 BOX, UNIT-DOSE 1 10 in 1 BLISTER PACK 2 NDC:63044-153-01 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/10/2005 Labeler - Nnodum Pharmaceuticals (960457273) Establishment Name Address ID/FEI Business Operations Contract Pharmacal Corporation 057795122 MANUFACTURE