Label: VILAZODONE HYDROCHLORIDE tablet
- NDC Code(s): 68788-8734-3, 68788-8735-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 72205-261, 72205-262
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VILAZODONE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for VILAZODONE HYDROCHLORIDE TABLETS.
VILAZODONE HYDROCHLORIDE tablets, for oral use
Initial U.S. Approval: 2011
- •
- Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric and young adult patients (5.1)
- •
- Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors (5.1)
- •
- Vilazodone hydrochloride tablets is not approved for use in pediatric patients (8.4)
INDICATIONS AND USAGE
Vilazodone hydrochloride tablets are indicated for the treatment of major depressive disorder (MDD) in adults (1).
DOSAGE AND ADMINISTRATION
- •
- Recommended target dosage: 20 mg to 40 mg once daily with food (2.1, 12.3).
- •
- To titrate: start with initial dosage of 10 mg once daily for 7 days, followed by 20 mg once daily. The dose may be increased
up to 40 mg once daily after a minimum of 7 days between dosage increases (2.1). - •
- Prior to initiating vilazodone hydrochloride, screen for bipolar disorder (2.2, 5.4).
- •
- When discontinuing vilazodone hydrochloride, reduce dosage gradually (2.4, 5.5).
DOSAGE FORMS AND STRENGTHS
Tablets: 10 mg, 20 mg and 40 mg (3)
CONTRAINDICATIONS
- •
- Concomitant use of monoamine oxidase inhibitors (MAOIs), or use within 14 days of stopping MAOIs (4).
WARNINGS AND PRECAUTIONS
- •
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents (e.g., SSRI, SNRI, triptans, amphetamines), but also when taken alone. If it occurs, discontinue vilazodone hydrochloride and initiate supportive treatment (5.2).
- •
- Increased Risk of Bleeding: Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may increase this risk (5.3).
- •
- Activation of Mania/Hypomania: Screen patients for bipolar disorder (5.4).
- •
- Seizures: Can occur with treatment. Use with caution in patients with a seizure disorder (5.6).
- •
- Angle Closure Glaucoma: Avoid use of antidepressants, including vilazodone hydrochloride, in patients with untreated anatomically narrow angles. (5.7).
- •
- Sexual Dysfunction: Vilazodone hydrochloride may cause symptoms of sexual dysfunction (5.9).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo): diarrhea, nausea, vomiting, and insomnia (6).To report SUSPECTED ADVERSE REACTIONS, contact Novadoz Pharmaceuticals LLC at 1-855-668-2369 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- CYP3A4 Inhibitors: The vilazodone hydrochloride dose should not exceed 20 mg once daily when co-administered with strong CYP3A4 inhibitors (2.4, 7).
- •
- CYP3A4 Inducers: Consider increasing vilazodone hydrochloride dosage by 2-fold, up to 80 mg once-daily over 1 to 2 weeks when used concomitantly with strong CYP3A4 inducers for greater than 14 days (2.4, 7).
USE IN SPECIFIC POPULATIONS
Pregnancy: Third trimester use may increase risk for persistent pulmonary hypertension and withdrawal in the newborn (8.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Treatment of Major Depressive Disorder
2.2 Screen for Bipolar Disorder Prior to Starting Vilazodone Hydrochloride
2.3 Switching to or from a Monoamine Oxidase Inhibitor Antidepressant
2.4 Dosage Adjustments with CYP3A4 Inhibitors or Inducers
2.5 Discontinuing Treatment with Vilazodone Hydrochloride
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behavior in Adolescents and Young Adults
5.2 Serotonin Syndrome
5.3 Increased Risk of Bleeding
5.4 Activation of Mania or Hypomania
5.5 Discontinuation Syndrome
5.6 Seizures
5.7 Angle-Closure Glaucoma
5.8 Hyponatremia
5.9 Sexual Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions With Vilazodone Hydrochloride
7.2 Drugs Having No Clinically Important Interactions With Vilazodone Hydrochloride
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Other Patient Populations
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse and Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions ( 5.1 )]. Vilazodone hydrochloride tablets is not approved for use in pediatric patients [see Use in Specific Populations ( 8.4)].
-
1 INDICATIONS AND USAGE
Vilazodone hydrochloride tablets are indicated for the treatment of major depressive disorder (MDD) in adults [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Treatment of Major Depressive Disorder
The recommended target dosage for vilazodone hydrochloride is 20 mg to 40 mg orally once daily with food [see Clinical Pharmacology (12.3), Clinical Studies (14)]. To achieve the target dosage, titrate vilazodone hydrochloride as follows:
- •
- Start with an initial dosage of 10 mg once daily with food for 7 days,
- •
- Then increase to 20 mg once daily with food.
- •
- The dose may be increased up to 40 mg once daily with food after a minimum of 7 days between dosage increases.
If a dose is missed, it should be taken as soon as the patient remembers. If it is almost time for the next dose, the patient should skip the missed dose and take the next dose at the regular time. Two doses should not be taken at the same time.
2.2 Screen for Bipolar Disorder Prior to Starting Vilazodone Hydrochloride
Prior to initiating treatment with vilazodone hydrochloride tablets or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.4)].
2.3 Switching to or from a Monoamine Oxidase Inhibitor Antidepressant
At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of vilazodone hydrochloride. In addition, at least 14 days must elapse after stopping vilazodone hydrochloride before starting an MAOI antidepressant [see Contraindications (4), Warnings and Precautions (5.2)].
2.4 Dosage Adjustments with CYP3A4 Inhibitors or Inducers
Patients receiving concomitant CYP3A4 inhibitors:
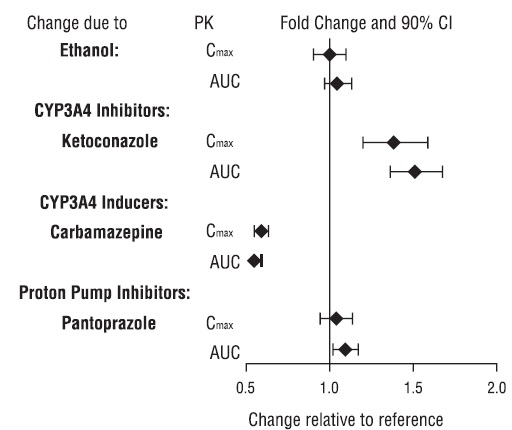
During concomitant use of a strong CYP3A4 inhibitor (e.g., itraconazole, clarithromycin, voriconazole), the vilazodone hydrochloride dose should not exceed 20 mg once daily. The original vilazodone hydrochloride dose level, can be resumed when the CYP3A4 inhibitor is discontinued [see Drug Interactions (7)].
Patients receiving concomitant CYP3A4 inducers:
Based on clinical response, consider increasing the dosage of vilazodone hydrochloride by 2-fold, up to a maximum 80 mg once daily, over 1 to 2 weeks in patients taking strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin) for greater than 14 days. If CYP3A4 inducers are discontinued, gradually reduce the vilazodone hydrochloride dosage to its original level over 1 to 2 weeks [see Drug Interactions (7)].2.5 Discontinuing Treatment with Vilazodone Hydrochloride
Adverse reactions may occur upon discontinuation of vilazodone hydrochloride [see Warnings and Precautions (5.5)]. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible. Vilazodone hydrochloride should be down tapered from the 40 mg once daily dose to 20 mg once daily for 4 days, followed by 10 mg once daily for 3 days. Patients taking vilazodone hydrochloride 20 mg once daily should be tapered to 10 mg once daily for 7 days.
-
3 DOSAGE FORMS AND STRENGTHS
Vilazodone Hydrochloride Tablets are available as 10 mg, 20 mg and 40 mg film-coated tablets.
10 mg: Pink colored, ellipse shaped, biconvex, film coated tablets, debossed with “MV” on one side and “14” on other side, free from physical defects.
20 mg: Orange colored, ellipse shaped, biconvex, film coated tablets, debossed with “MV” on one side and “15” on other side, free from physical defects.
40 mg: Blue colored, ellipse shaped, biconvex, film coated tablets, debossed with “MV” on one side and “16” on other side, free from physical defects -
4 CONTRAINDICATIONS
Vilazodone hydrochloride tablets are contraindicated in:
- •
- Patients taking, or within 14 days of stopping, monoamine oxidase inhibitors (MAOIs), including MAOIs such as linezolid or intravenous methylene blue, because of an increased risk of serotonin syndrome [see Warnings and Precautions (5.2),Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behavior in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients, and over 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater in antidepressant-treated patients than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
Table 1: Risk Differences of the Number of Patients with Suicidal Thoughts or Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
Age Range
(years)
Drug-Placebo Difference in Number of Patients with Suicidal Thoughts or Behaviors per 1000
Patients Treated
Increases Compared to Placebo
<18
14 additional patients
18-24
5 additional patients
Decreases Compared to Placebo
25-64
1 fewer patient
≥65
6 fewer patients
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing vilazodone hydrochloride, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
Serotonin and norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitor (SSRIs), including vilazodone hydrochloride, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4) and Drug Interactions (7)]. Serotonin syndrome can also occur when these drugs are used alone. Symptoms of serotonin syndrome were noted in 0.1% of MDD patients treated with vilazodone hydrochloride in premarketing clinical trials.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of vilazodone hydrochloride with MAOIs is contraindicated. In addition, do not initiate vilazodone hydrochloride in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking vilazodone hydrochloride, discontinue vilazodone hydrochloride before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7.1)].
Monitor all patients taking vilazodone hydrochloride for the emergence of serotonin syndrome. Discontinue treatment with vilazodone hydrochloride and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of vilazodone hydrochloride with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.5.3 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including vilazodone hydrochloride, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the risk of bleeding associated with the concomitant use of vilazodone hydrochloride and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor coagulation indices when initiating, titrating, or discontinuing vilazodone hydrochloride.5.4 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with vilazodone hydrochloride or another antidepressant may precipitate a mixed/manic episode. In controlled clinical trials, patients with bipolar disorder were excluded; however, symptoms of mania or hypomania were reported in 0.1% of undiagnosed patients treated with vilazodone hydrochloride. Prior to initiating treatment with vilazodone hydrochloride, screen patients for any personal or family history of bipolar disorder, mania, or hypomania [see Dosage and Administration (2.2)].
5.5 Discontinuation Syndrome
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible [see Dosage and Administration (2.5)].
5.6 Seizures
Vilazodone hydrochloride has not been systematically evaluated in patients with a seizure disorder. Patients with a history of seizures were excluded from clinical studies. Vilazodone hydrochloride should be prescribed with caution in patients with a seizure disorder.
5.7 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including vilazodone hydrochloride may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including vilazodone hydrochloride, in patients with untreated anatomically narrow angles.
5.8 Hyponatremia
Hyponatremia may occur as a result of treatment with SNRIs and SSRIs, including vilazodone hydrochloride. Cases of serum sodium lower than 110 mmol/L have been reported. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue vilazodone hydrochloride and institute appropriate medical intervention. Elderly patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs and SNRIs [see Use in Specific Populations (8.5)].5.9 Sexual Dysfunction
Use of SSRIs, including vilazodone hydrochloride, may cause symptoms of sexual dysfunction [see Adverse Reactions (6.1)]. In male patients, SSRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of vilazodone hydrochloride and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment. -
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions (5.1)].
- •
- Serotonin Syndrome [see Warnings and Precautions (5.2)].
- •
- Increased Risk of Bleeding [see Warnings and Precautions (5.3)].
- •
- Activation of Mania or Hypomania [see Warnings and Precautions (5.4)].
- •
- Discontinuation Syndrome [see Warnings and Precautions (5.5)].
- •
- Seizures [see Warnings and Precautions (5.6)].
- •
- Angle-Closure Glaucoma [see Warnings and Precautions (5.7)].
- •
- Hyponatremia [see Warnings and Precautions (5.8)].
- •
- Sexual Dysfunction [see Warnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions and varying lengths of time, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice.
The most commonly observed adverse reactions in vilazodone hydrochloride-treated patients with major depressive disorder (MDD) in placebo-controlled studies (incidence ≥ 5% and at least twice the rate of placebo) were diarrhea, nausea, vomiting, and insomnia.Patient Exposure
The safety of vilazodone hydrochloride was evaluated in 3,007 patients (18 to 70 years of age) diagnosed with MDD who participated in clinical studies, representing 676 patient-years of exposure. In an open-label 52 week study at 40 mg daily, 599 patients were exposed to vilazodone hydrochloride for a total of 348 patient-years.
The adverse reaction information presented below was derived from studies of vilazodone hydrochloride 20 mg and 40 mg daily in patients with MDD including:
• Four placebo-controlled 8 to 10-week studies in 2,233 patients, including 1,266 vilazodone hydrochloride-treated patients; and
• An open-label 52-week study of 599 vilazodone hydrochloride-treated patients.
These studies included a titration period of 10 mg daily for 7 days, followed by 20 mg daily for 7 days or to 40 mg daily over 2 weeks. In these clinical trials, vilazodone hydrochloride was administered with food.
Adverse reactions reported as reasons for discontinuation of treatment
In these studies, 7.3% of the vilazodone hydrochloride-treated patients discontinued treatment due to an adverse reaction, compared with 3.5% of placebo-treated patients. The most common adverse reaction leading to discontinuation in at least 1% of the vilazodone hydrochloride-treated patients in the placebo-controlled studies was nausea (1.4%).
Common adverse reactions in placebo-controlled MDD studies
Table 2 shows the incidence of common adverse reactions occurring in ≥ 2% of vilazodone hydrochloride-treated patients and greater than the rate of placebo-treated patients in MDD Studies. There were no dose-related adverse reactions between 20 mg and 40 mg reported.
Table 2: Common Adverse Reactions Occurring in ≥ 2% of Vilazodone Hydrochloride-treated Patients and Greater than the Rate of Placebo-Treated Patients
System Organ Class
Preferred Term
Placebo
N=967
Vilazodone Hydrochloride
20 mg/day
N=288
Vilazodone Hydrochloride
40 mg/day
N=9781 Includes abdominal discomfort, abdominal pain upper, and abdominal pain.
2 Includes headache and tension headache
3 Includes restlessness, akathisia, and restless legs syndrome
Sexual adverse reactions are presented in Table 3
Gastrointestinal disorders
Diarrhea
10%
26%
29%
Nausea
7%
22%
24%
Dry mouth
5%
8%
7%
Vomiting
2%
4%
5%
Abdominal pain1
3%
7%
4%
Dyspepsia
2%
2%
3%
Flatulence
1%
3%
3%
Gastroenteritis
1%
1%
2%
Abdominal distension
1%
2%
1%
Nervous system disorders
Headache2
14%
15%
14%
Dizziness
5%
6%
8%
Somnolence
2%
4%
5%
Paresthesia
1%
1%
2%
Psychiatric disorders
Insomnia
2%
7%
6%
Abnormal dreams
2%
2%
3%
Restlessness3
1%
2%
3%
General disorders
Fatigue
3%
4%
3%
Cardiac disorders
Palpitations
<1%
1%
2%
Metabolism and nutrition disorders
Increased appetite
1%
1%
3%
Musculoskeletal and connective tissue disorders
Arthralgia
1%
2%
1%
Investigations
Increased weight
1%
1%
2%
Sexual adverse reactions
Table 3 displays the most common sexual adverse reactions in the placebo-controlled MDD studies.
Table 3: Common Sexual Adverse Reactions Occurring in ≥ 2% of Vilazodone Hydrochloride-treated Patients and Greater than the Rate of Placebo-Treated Patients
− Not applicable
*Includes abnormal orgasm and anorgasmia
Preferred TermMales
Females
Placebo
N=416
Vilazodone Hydrochloride 20 mg/day
N=122
Vilazodone Hydrochloride
40 mg/day
N=417
Placebo
N=551
Vilazodone Hydrochloride 20 mg/day
N=166
Vilazodone Hydrochloride
40 mg/day
N=561Abnormal Orgasm*
<1%
2%
2%
0%
1%
1%
Erectile dysfunction
1%
0%
3%
-
-
-
Libido decreased
<1%
3%
4%
<1%
2%
2%
Ejaculation disorder
0%
1%
2%
-
-
-
Other adverse reactions observed in clinical studies
The following list does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients:
Cardiac disorders: infrequent: ventricular extrasystoles
Eye disorders: infrequent: dry eye, vision blurred, rare: cataracts
Nervous System: frequent: sedation, tremor; infrequent: migraine
Psychiatric disorders: infrequent: panic attack
Skin and subcutaneous tissue disorders: infrequent: hyperhidrosis, night sweats6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of vilazodone hydrochloride. Because these
reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish
a causal relationship to drug exposure. Reports of adverse reactions temporally associated with vilazodone hydrochloride that have
been received since market introduction and that are not listed above include the following:
General Disorders and Administration Site Conditions: irritability
Nervous System Disorders: sleep paralysis
Psychiatric Disorders: hallucinations, suicide attempt, suicidal ideation
Skin and subcutaneous tissue disorders: rash, generalized rash, urticaria, drug eruption
Gastrointestinal System: acute pancreatitis -
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions With Vilazodone Hydrochloride
Table 1: Clinically Important Drug Interactions with Vilazodone Hydrochloride
Concomitant Drug Name or Drug Class
Clinical Rationale
Clinical RecommendationMonoamine Oxidase Inhibitors (MAOIs)
The concomitant use of MAOIs and serotonergic drugs including vilazodone hydrochloride increases the risk of serotonin syndrome.
Vilazodone hydrochloride is contraindicated in patients taking MAOIs, including MAOIs such as linezolid or intravenous methylene blue[see Contraindications (4), Dosage and Administration (2.3), and Warnings and Precautions (5.2)].
Other Serotonergic Drugs
The concomitant use of serotonergic drugs including vilazodone hydrochloride and other serotonergic drugs increases the risk of serotonin syndrome.
Monitor patients for signs and symptoms of serotonin syndrome, particularly during vilazodone hydrochloride initiation. If serotonin syndrome occurs, consider discontinuation of vilazodone hydrochloride and/or concomitant serotonergic drugs [see Warnings and Precautions (5.2)].
Antiplatelet Agents and Anticoagulants
Serotonin release by platelets plays an important role in hemostasis. The concurrent use of an antiplatelet agent or anticoagulant with vilazodone hydrochloride may potentiate the risk of bleeding.
Inform patients of the increased risk of bleeding with the concomitant use of vilazodone hydrochloride and antiplatelet agents and anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio (INR) when initiating, titrating, or discontinuing vilazodone hydrochloride [see Warnings and Precautions (5.3)].
Strong CYP3A4 Inhibitors (e.g., itraconazole, clarithromycin, voriconazole)
The concomitant use of vilazodone hydrochloride and strong CYP3A4 inhibitors increased the exposure of vilazodone compared to the use of vilazodone hydrochloride alone [see Clinical Pharmacology (12.3)].
The vilazodone hydrochloride dose should not exceed 20 mg once daily with the concomitant use of a strong CYP3A4 inhibitor [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
Strong CYP3A4 Inducers (e.g.,
carbamazepine, phenytoin, rifampin)The concomitant use of vilazodone hydrochloride and strong CYP3A4 inducers decreased the exposure of vilazodone compared to the use of vilazodone hydrochloride alone [see Clinical Pharmacology (12.3)].
Based on clinical response, consider increasing the dosage of vilazodone hydrochloride, over 1 to 2 weeks inpatients taking strong CYP3A4 inducers for greater than 14 days [see Dosage and Administration (2.4),Clinical Pharmacology (12.3)].
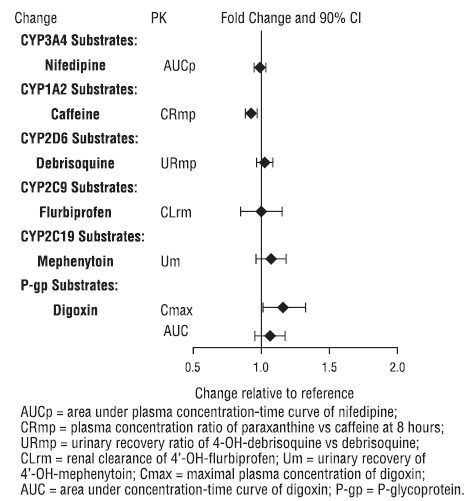
Digoxin
Digoxin is a narrow therapeutic index drug. Concomitant use of vilazodone hydrochloride increased digoxin concentrations [see Clinical Pharmacology (12.3)].
Measure serum digoxin concentrations before initiating concomitant use of vilazodone hydrochloride. Continue monitoring and reduce digoxin dose as necessary.
7.2 Drugs Having No Clinically Important Interactions With Vilazodone Hydrochloride
Based on pharmacokinetic studies, no dosage adjustment is required for drugs that are substrates of CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and/or P-glycoprotein (except narrow therapeutic index drugs, e.g., digoxin), when vilazodone hydrochloride is administered concomitantly [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405- 6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants.
Risk Summary
There are no adequate and well-controlled studies of vilazodone hydrochloride in pregnant women. The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies. In animal reproduction studies, oral administration of vilazodone during the period of organogenesis at doses up to 48 and 17 times the maximum recommended human dose (MRHD) in rats and rabbits, respectively, resulted in decreased fetal body weight gain and delayed skeletal ossification but no teratogenic effects were observed. Decreased fetal body weight and delayed skeletal ossification were not observed at doses up to 10 and 4 times the MRHD in rats and rabbits, respectively [see Data].
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
A prospective, longitudinal study followed 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. The women who discontinued antidepressants during pregnancy were more likely to experience a relapse of major depression than women who continued antidepressants. Consider the risks of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Fetal/Neonatal adverse reactions
Exposure to SSRIs and SNRIs, including vilazodone hydrochloride, in late pregnancy may lead to an increased risk for neonatal complications requiring prolonged hospitalization, respiratory support, and tube feeding, and/or persistent pulmonary hypertension of the newborn (PPHN). Monitor neonates who were exposed to vilazodone hydrochloride in the third trimester of pregnancy for PPHN and drug discontinuation syndrome [see Data)].
Data
Human Data
Third Trimester Exposure
Neonates exposed to SSRIs or SNRIs late in the third trimester, have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. These findings are based on post-marketing reports. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. In some cases, the clinical picture was consistent with serotonin syndrome [see Warnings and Precautions (5.2)].
Exposure during late pregnancy to SSRIs may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1 to 2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality. In a retrospective case-control study of 377 women whose infants were born with PPHN and 836 women whose infants were born healthy, the risk for developing PPHN was approximately six-fold higher for infants exposed to SSRIs after the 20th week of gestation compared to infants who had not been exposed to antidepressants during pregnancy. A study of 831,324 infants born in Sweden in 1997-2005 found a PPHN risk ratio of 2.4 (95% CI 1.2 to 4.3) associated with patient-reported maternal use of SSRIs “in early pregnancy” and a PPHN risk ratio of 3.6 (95% CI 1.2 to 8.3) associated with a combination of patient-reported maternal use of SSRIs “in early pregnancy” and an antenatal SSRI prescription “in later pregnancy.”
Animal Data
No teratogenic effects were observed when vilazodone was given to pregnant rats or rabbits during the period of organogenesis at oral doses up to 200 and 36 mg/kg/day, respectively. These doses are 48 and 17 times, in rats and rabbits, respectively, the maximum recommended human dose (MRHD) of 40 mg on a mg/m2 basis. Fetal body weight gain was reduced, and skeletal ossification was delayed in both rats and rabbits at these doses; these effects were not observed at doses up to 10 times the MRHD in rats or 4 times the MRHD in rabbits.
When vilazodone was administered to pregnant rats at an oral dose of 30 times the MRHD during the period of organogenesis and throughout pregnancy and lactation, the number of live born pups was decreased. There was an increase in early postnatal pup mortality, and among surviving pups there was decreased body weight, delayed maturation, and decreased fertility in adulthood. There was some maternal toxicity at this dose. These effects were not seen at 6 times the MRHD.8.2 Lactation
Risk Summary
There are no data on the presence of vilazodone in human milk, the effects of vilazodone on the breastfed infant, or the effects of the drug on milk production. However, vilazodone is excreted in rat milk [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for vilazodone hydrochloride and any potential adverse effects on the breastfed child from vilazodone hydrochloride or from the underlying maternal condition.
Data
Animal Data
Administration of vilazodone to lactating rats at an oral dose of 30 times the maximum recommended human dose (MRHD), resulted in early postnatal pup mortality, and among surviving pups there was decreased body weight and delayed maturation.8.4 Pediatric Use
The safety and effectiveness of vilazodone hydrochloride tablets have not been established in pediatric patients for the treatment of MDD.
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients[see Boxed Warning, Warnings and Precautions (5.1), and Adverse Reactions (6.2)].
Juvenile Animal Toxicity Data
In a juvenile animal study, male and female rats were treated with vilazodone (10, 50, and 200 mg/kg/day) starting on postnatal day (PND) 21 through 90. A delay in the age of attainment of vaginal patency (i.e. sexual maturation) was observed in females starting at 50 mg/kg/day with a No Observed Adverse Effect Level (NOAEL) of 10 mg/kg/day. Adverse behavioral effects (lack of habituation in an acoustic startle test) were observed in males at 200 mg/kg and females starting at 50 mg/kg both during drug treatment and the recovery periods. The NOAEL for this finding was 50 mg/kg for males and 10 mg/kg for females. An 8% decrease in femur mineral density was observed in female rats at 200 mg/kg, compared to the control group. The NOAEL for this finding was 50 mg/kg.
Pediatric information describing a clinical study in which efficacy was not demonstrated is approved for Allergan’s Viibryd (vilazodone hydrochloride) tablets. However, due to Allergan’s marketing exclusivity rights, this drug product is not labeled with that information.8.5 Geriatric Use
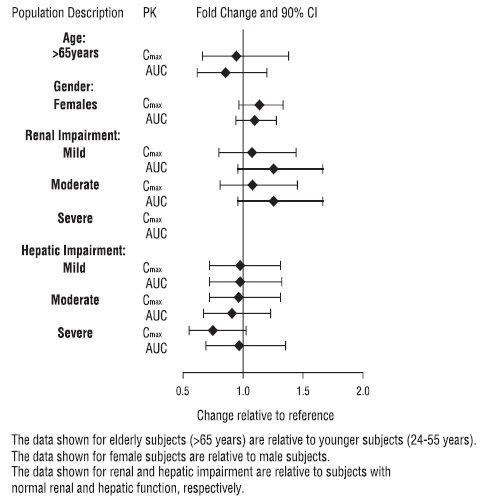
Based on a pharmacokinetic study, no dosage adjustment of vilazodone hydrochloride is recommended on the basis of age (see Figure 3). Results from pharmacokinetic study of a single 20 mg vilazodone hydrochloride dose in geriatric subjects (> 65 years-old) vs. younger subjects (24 to 55 years-old) demonstrated that the pharmacokinetics were generally similar between the two age groups [see Clinical Pharmacology (12.3)].
Clinical studies of vilazodone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Of the 3,007 patients in clinical studies with vilazodone hydrochloride, 65 (2.2%) were 65 years of age or older, and 378 (12.6%) were 55 to 64 years of age. In general, dose selection for an elderly patient should be conservative, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Serotonergic antidepressants have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions (5.8)]. No other differences in adverse reactions were observed between geriatric and younger patients.8.6 Use in Other Patient Populations
No dosage adjustment of vilazodone hydrochloride is necessary on the basis of gender, renal function (mild to severe renal impairment, glomerular filtration rate: 15 to 90 mL/minute), or hepatic function (mild to severe hepatic impairment, Child-Pugh score: 5 to 15 [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse and Dependence
Vilazodone hydrochloride has been systematically studied in animals and did not demonstrate abuse or dependence potential. While vilazodone hydrochloride has not been systematically studied in humans for its potential for abuse, there was no suggested evidence of drug-seeking behavior in the clinical studies.
-
10 OVERDOSAGE
There is limited clinical trial experience regarding human overdose with vilazodone hydrochloride. The adverse reactions associated with overdose of vilazodone hydrochloride at doses of 200 to 280 mg (5 to 7 times the recommended dosage) as observed in clinical trials included serotonin syndrome, lethargy, restlessness, hallucinations, and disorientation.
For current information on the management of poisoning or overdose, contact a poison control center at 1-800-222-1222. No specific antidotes for vilazodone are known. Removal of vilazodone by dialysis has not been studied; however, the high volume of distribution of vilazodone suggests that dialysis will not be effective in reducing vilazodone plasma concentrations. -
11 DESCRIPTION
Vilazodone hydrochloride tablets for oral administration contain polymorph Form IV vilazodone hydrochloride (HCl), a selective serotonin reuptake inhibitor and a 5HT1A receptor partial agonist.
Vilazodone Hydrochloride is 2-benzofurancarboxamide, 5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-, hydrochloride with a molecular formula of C26H27N5O2.HCl. Its molecular weight is 479.99 g/mol. The structural formula is: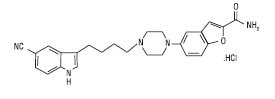
Vilazodone hydrochloride tablets are available as 10 mg, 20 mg, and 40 mg film-coated tablets containing 10 mg, 20 mg, and 40 mg of vilazodone HCl, respectively.
In addition to the active ingredient, vilazodone hydrochloride tablets contain the following inactive ingredients: colloidal silicon dioxide, FD&C Blue #1 Aluminum Lake (40 mg only), Ferric Oxide Red (10 mg only) and FD&C Yellow #6 Aluminum Lake (20 mg only), lactose monohydrate, macrogol (polyethylene glycol), magnesium stearate, microcrystalline cellulose, opadry II blue (for 40 mg), opadry II pink (for 10 mg) and opadry II orange (for 20 mg), polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
The mechanism of action of vilazodone in the treatment of major depressive disorder is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS through selective inhibition of serotonin reuptake. Vilazodone is also a partial agonist at serotonergic 5-HT1A receptors; however, the net result of this action on serotonergic transmission and its role in vilazodone’s antidepressant effect are unknown.
12.2 Pharmacodynamics
Vilazodone binds with high affinity to the serotonin reuptake site (Ki= 0.1 nM), but not to the norepinephrine (Ki=56 nM) or dopamine (Ki=37 nM) reuptake sites. Vilazodone potently and selectively inhibits reuptake of serotonin (IC50= 1.6 nM). Vilazodone also binds selectively with high affinity to 5-HT1A receptors (IC50=2.1 nM) and is a 5-HT1A receptor partial agonist.
Cardiac Electrophysiology
Treatment with vilazodone hydrochloride did not prolong the QTc interval. The effect of vilazodone hydrochloride [20, 40, 60, and 80 mg (2 times the recommended dosage)] on the QTc interval was evaluated in a randomized, placebo-, and active-controlled (moxifloxacin 400 mg), parallel-group, thorough QTc study in 157 healthy subjects. The study demonstrated an ability to detect small effects. The upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc interval was below 10 msec, based on the individual correction method (QTcI). Thus, at doses of 2 times the recommended dosage, vilazodone hydrochloride did not prolong the QTc interval to a clinically relevant extent.12.3 Pharmacokinetics
Vilazodone activity is due primarily to the parent drug. The pharmacokinetics of vilazodone (5 mg to 80 mg) are dose-proportional. Accumulation of vilazodone after administration of single vilazodone hydrochloride doses did not vary with dose, and steady-state was achieved in about 3 days. Elimination of vilazodone is primarily by hepatic metabolism with a terminal half-life of approximately 25 hours. At steady-state, after daily dosing of vilazodone hydrochloride 40 mg under fed conditions, the mean Cmax value was 156 ng/mL, and the mean AUC (0-24 hours) value was 1645 ng·h/mL.
Absorption
Vilazodone concentrations peaked at a median of 4 to 5 hours (Tmax) after vilazodone hydrochloride administration and declined with a terminal half-life of approximately 25 hours. The absolute bioavailability of vilazodone was 72% with food. Vilazodone AUC and Cmax in the fasted state can be decreased by approximately 50% and 60%, respectively, compared to the fed state. Administration without food can result in inadequate drug concentrations and may reduce effectiveness.
Co-administration of vilazodone hydrochloride with ethanol or with a proton pump inhibitor (pantoprazole) did not affect the rate or extent of vilazodone absorption. In addition, neither the Tmax nor terminal elimination rate of vilazodone was altered by co-administration with either pantoprazole or ethanol.
Absorption is decreased by approximately 25% if vomiting occurs within 7 hours of ingestion; no replacement dose is needed.
Distribution
Vilazodone is widely distributed and approximately 96 to 99% protein-bound. Administration of vilazodone hydrochloride to a patient taking another drug that is highly protein bound may cause increased free concentrations of the other drug, because vilazodone is highly bound to plasma protein. The interaction between vilazodone and other highly protein-bound drugs has not been evaluated.
Metabolism and Elimination
Vilazodone hydrochloride is extensively metabolized through CYP and non-CYP pathways (possibly by carboxylesterase), with only 1% of the dose recovered in the urine and 2% of the dose recovered in the feces as unchanged vilazodone. CYP3A4 is primarily responsible for its metabolism among CYP pathways, with minor contributions from CYP2C19 and CYP2D6.
Drug Interaction Studies
Figure 1 below includes the impact of other drugs on the pharmacokinetics of vilazodone [see Drug Interactions (7)].
Figure 1. Effect of Other Drugs on Vilazodone Pharmacokinetics
In vitro studies indicate that vilazodone is unlikely to inhibit or induce the metabolism of substrates for CYP1A1, 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, 3A4 or 3A5, except for CYP2C8. The effect of vilazodone on CYP2C8 activity has not been tested in vivo.
Figure 2 below includes the impact of vilazodone on the pharmacokinetics of other drugs in vivo.
Figure 2. Impact of Vilazodone on Other Drug Pharmacokinetics
Studies in Specific Populations:
The presence of mild to severe renal impairment or mild to severe hepatic impairment did not affect the apparent clearance of vilazodone (see Figure 3). There were no pharmacokinetic differences of vilazodone in geriatric patients compared to younger patients, or between males and females (see Figure 3).
Figure 3: Impact of Intrinsic Factors on Vilazodone Pharmacokinetics
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in which B6C3F1 mice and Wistar rats were given oral doses of vilazodone up to 135 and 150 mg/kg/day, respectively, for 2 years. These doses are approximately 16.5 and 36 times the maximum recommended human dose (MRHD) of 40 mg, respectively, on a mg/m2 basis.
In mice, the incidence of hepatocellular carcinomas was increased in males at 16.5 times the MRHD; this finding was not observed at 5.5 times the MRHD. The incidence of malignant mammary gland tumors was numerically increased in females at 5.5 and 16.5 times the MRHD, with statistical significance at 16.5 the MRHD; this finding was not observed at 1.8 times the MRHD. Elevated prolactin levels were observed in a 2-week study of vilazodone administered at 5.5 and 33 times the MRHD. Increases in prolactin levels are known to cause mammary tumors in rodents.
In the rat study, vilazodone was not carcinogenic in either sex at doses up to 36 times the MRHD.
Mutagenesis
Vilazodone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test). Vilazodone was negative in the in vitro V79/HGRPT mammalian cell forward mutation assay. Vilazodone was clastogenic in two in vitro mammalian cell chromosome aberration assays. However, vilazodone was negative for clastogenic activity in both an in vivo rat bone marrow chromosome aberration assay and a micronucleus test. Vilazodone was also negative in an in vivo/in vitro unscheduled DNA synthesis assay in rats.
Impairment of Fertility
Treatment of rats with vilazodone at a dose of 125 mg/kg, which is 30 times the MRHD of 40 mg on a mg/m2 basis, caused impairment of male fertility with no effect on female fertility. Impaired male fertility was not observed at 6 times the MRHD. -
14 CLINICAL STUDIES
The efficacy of vilazodone hydrochloride as a treatment for major depressive disorder was demonstrated in four multicenter, randomized, double-blind, placebo-controlled studies in adult (18 to 70 years of age) outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD. Three 8-week studies evaluated the efficacy of vilazodone hydrochloride 40 mg (Studies 1 to 3) and one 10-week study (Study 4) evaluated the efficacy of vilazodone hydrochloride 20 mg and 40 mg (see Table 5). In these studies, patients were randomized to either 20 mg or 40 mg, or placebo once daily with food. Patients were either titrated over 1 week to a dose of 20 mg daily or over 2 weeks to a dose of 40 mg once daily of vilazodone hydrochloride with food. Vilazodone hydrochloride was superior to placebo in the improvement of depressive symptoms as measured by the change from baseline to endpoint visit in the Montgomery-Asberg Depression Rating Scale (MADRS) total score for both doses. The MADRS is a ten-item, clinician-rated scale used to assess severity of depressive symptoms. Scores on the MADRS range from 0 to 60, with higher scores indicating more severe depression. Clinical Global Impression - Severity (CGI-S) was evaluated in Studies 3 and 4. Vilazodone hydrochloride 20 mg and 40 mg demonstrated superiority over placebo as measured by improvement in CGI-S score.
Table 2: Summary of Results for the Primary Efficacy Endpoint - MADRS Total Score SD = standard deviation; SE = standard error; LS Mean = least-square mean; CI = confidence interval
a based on patients who took study medication and had baseline and post baseline MADRS assessments
b difference (drug minus placebo) in least-square mean change from baseline to endpoint
* All vilazodone hydrochloride treatment dose groups remained statistically significant compared with placebo after adjusting for multiplicity
Study
NumberTreatment Group
Number of Patients a
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference b
(95% CI)Study 1
Vilazodone hydrochloride 40 mg/day
198
30.8 (3.90)
-12.9 (0.77)
-3.2 (-5.2, -1.3)
Placebo
199
30.7 (3.93)
-9.6 (0.76)
Study 2
Vilazodone hydrochloride 40 mg/day
231
31.9 (3.50)
-13.3 (0.90)
-2.5 (-4.4, -0.6)
Placebo
232
32.0 (3.63)
-10.8 (0.90)
Study 3
Vilazodone hydrochloride 40 mg/day
253
30.7 (3.3)
-16.1 (0.64)
-5.1 (-6.9, -3.3)
Placebo
252
30.9 (3.3)
-11.0 (0.65)
Vilazodone hydrochloride 20 mg/day*
288
31.3 (3.5)
-17.3 (0.63)
-2.6 (-4.3, -0.8)
Study 4
Vilazodone hydrochloride 40 mg/day*
284
31.2 (3.8)
-17.6 (0.65)
-2.8 (-4.6, -1.1)
Placebo
281
31.4 (3.8)
-14.8 (0.62)
Baseline demographics information were generally similar across all treatment groups. Examination of population subgroups based on age (there were few patients over 65), gender and race did not reveal any clear evidence of differential responsiveness.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Vilazodone hydrochloride tablets are supplied in the following configurations:
20 mg: Orange colored, ellipse shaped, biconvex, film coated tablets, debossed with “MV” on one side and “15” on other side,
68788-8734-3 30-count bottles
40 mg: Blue colored, ellipse shaped, biconvex, film coated tablets, debossed with “MV” on one side and “16” on other side.68788-8735-330-count bottles
Vilazodone hydrochloride tablets should be stored at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dosage is adjusted up or down and instruct them to report such symptoms to the healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Dosage and Administration
Instruct patients to take vilazodone hydrochloride with food and to follow prescribed dosage instructions [see Dosage and Administration (2.1, 2.3, 2.4, 2.5)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of vilazodone hydrochloride with other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Patients should contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7)].
Increased Risk of Bleeding
Inform patients about the concomitant use of vilazodone hydrochloride tablets with aspirin, NSAIDs, other antiplatelet drugs, warfarin, or other anticoagulants because the combined use of drugs that interfere with serotonin reuptake (e.g., vilazodone hydrochloride) and these medications has been associated with an increased risk of bleeding. Advise them to inform their health care providers if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding[see Warnings and Precautions (5.3)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions (5.4)].
Discontinuation Syndrome
Advise patients not to abruptly discontinue vilazodone hydrochloride tablets and to discuss any tapering regimen with their healthcare provider. Adverse reactions can occur when vilazodone hydrochloride tablets are discontinued[see Warnings and Precautions (5.5 )].Seizures
Caution patients about using vilazodone hydrochloride if they have a history of a seizure disorder [see Warnings and Precautions (5.6)].
Sexual Dysfunction
Advise patients that use of vilazodone hydrochloride may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.9)].
Allergic Reactions
Advise patients to notify their healthcare provider if they develop an allergic reaction such as rash, hives, swelling, or difficulty breathing [see Adverse Reactions (6.2)].
Concomitant Medications
Advise patients to inform their health care providers if they are taking, or plan to take any prescription or over-the-counter medications since there is a potential for interactions[see Drug Interactions (7.1)].
Pregnancy- •
- Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with vilazodone hydrochloride tablets[see Use in Specific Populations (8.1)].
- •
- Advise patients that vilazodone hydrochloride tablets use late in pregnancy may lead to an increased risk for neonatal complications requiring prolonged hospitalization, respiratory support, tube feeding, and/or persistent pulmonary hypertension of the newborn (PPHN) [see Use in Specific Populations (8.1)].
- •
- Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to vilazodone hydrochloride tablets during pregnancy [see Use in Specific Populations (8.1)].
Manufactured by:
MSN Laboratories Private Limited
Telangana – 509 228,
INDIA
Distributed by:
Novadoz Pharmaceuticals LLC
Piscataway, NJ 08854-3714
Issued on: June 2023Repackaged By: Preferred Pharmaceuticals Inc.
-
MEDICATION GUIDE
Vilazodone Hydrochloride Tablets, for oral use
(vil-AZ-oh-done)What is the most important information I should know about vilazodone hydrochloride tablets?
Vilazodone hydrochloride tablets may cause serious side effects, including:- •
- Increased risk of suicidal thoughts or actions in some children, adolescents, and young adults. Vilazodone hydrochloride tablets and other antidepressant medicines may increase suicidal thoughts or actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed.Vilazodone hydrochloride tablets are not for use in children.
- •
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness) or have a history of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions?
- •
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- •
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- •
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider or get emergency medical help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you: - •
- attempts to commit suicide
- •
- acting on dangerous impulses
- •
- acting aggressive, being angry or violent
- •
- thoughts about suicide or dying
- •
- new or worse depression
- •
- new or worse anxiety
- •
- panic attacks
- •
- feeling agitated or restless
- •
- new or worse irritability
- •
- trouble sleeping (insomnia)
- •
- an extreme increase in activity or talking (mania)
- •
- other unusual changes in behavior or mood
What are vilazodone hydrochloride tablets?
Vilazodone hydrochloride tablets are a prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD) in adults. It is not known if vilazodone hydrochloride tablets are safe and effective for use in children for the treatment of MDD.
Who should not take vilazodone hydrochloride tablets?
Do not take vilazodone hydrochloride tablets if you:- •
- take a Monoamine Oxidase Inhibitor (MAOI)
- •
- have stopped taking an MAOI in the last 14 days
- •
- are being treated with the antibiotic linezolid or intravenous methylene blue
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for at least 14 days after you stop treatment with vilazodone hydrochloride.Before taking vilazodone hydrochloride tablets, tell your healthcare provider about all your medical conditions, including if you:
- •
- have or have a family history of suicide, depression, bipolar disorder, mania or hypomania
- •
- have or had bleeding problems
- •
- have or had seizures or convulsions
- •
- have high pressure in the eye (glaucoma)
- •
- have low sodium levels in your blood
- •
- drink alcohol
- •
- are pregnant or plan to become pregnant. Taking vilazodone hydrochloride tablets late in pregnancy may lead to an increased risk of certain problems in your newborn. Talk to your healthcare provider about the risks to your baby if you take vilazodone hydrochloride tablets during pregnancy. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with vilazodone hydrochloride tablets.
- •
- There is a pregnancy registry for females who are exposed to vilazodone hydrochloride pregnancy. The purpose of the registry is to collect information about the health of females exposed to vilazodone hydrochloride and their baby. If you become pregnant during treatment with vilazodone hydrochloride, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185.
- •
- are breastfeeding or plan to breastfeed. It is not known if vilazodone hydrochloride passes into breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with vilazodone hydrochloride tablets.
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Vilazodone hydrochloride and some medicines may affect each other causing possible serious side effects. Vilazodone hydrochloride may affect the way other medicines work and other medicines may affect the way vilazodone hydrochloride works.Especially tell your healthcare provider if you take:
- •
- MAOIs
- •
- medicines used to treat migraine headaches known as triptans
- •
- tricyclic antidepressants
- •
- fentanyl
- •
- lithium
- •
- tramadol
- •
- tryptophan
- •
- buspirone
- •
- amphetamines
- •
- St. John's Wort
- •
- medicines that can affect blood clotting such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) and warfarin
- •
- diuretics
- •
- medicines used to treat mood, anxiety, psychotic or thought disorders, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs)
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take vilazodone hydrochloride tablets with your other medicines.
Do not start or stop any other medicines during treatment vilazodone hydrochloride tablets without talking to your healthcare provider first. Stopping vilazodone hydrochloride tablets suddenly may cause you to have serious side effects. See, "What are the possible side effects of vilazodone hydrochloride tablets?"
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.How should I take vilazodone hydrochloride tablets?
- •
- Take vilazodone hydrochloride tablets exactly as your healthcare provider tell you to. Do not change your dose or stop taking vilazodone hydrochloride tablets without first talking to your healthcare provider.
- •
- Your healthcare provider may need to change the dose of vilazodone hydrochloride until it is the right dose for you.
- •
- Take vilazodone hydrochloride tablets 1 time each day with food.
- •
- If you miss a dose of vilazodone hydrochloride tablets, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of vilazodone hydrochloride tablets at the same time.
- •
- If you take too much vilazodone hydrochloride tablets, call your healthcare provider or poison control center at 1-800-222-1222 right away, or get emergency treatment right away.
What should I avoid while taking vilazodone hydrochloride tablets?
- •
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how vilazodone hydrochloride tablets affects you. Vilazodone hydrochloride tablets can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly.
- •
- Avoid drinking alcohol during treatment with vilazodone hydrochloride tablets.
What are the possible side effects of vilazodone hydrochloride tablets?
Vilazodone hydrochloride tablets may cause serious side effects, including:- •
- See, "What is the most important information I should know about vilazodone hydrochloride tablets?’’
- •
- Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome can happen when vilazodone hydrochloride tablets are taken with certain other medicines. See, "Who should not take vilazodone hydrochloride tablets?" Stop taking vilazodone hydrochloride tablets and call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of serotonin syndrome:
- •
- agitation
- •
- seeing or hearing things that are not real (hallucinations)
- •
- confusion
- •
- coma
- •
- fast heart beat
- •
- blood pressure changes
- •
- dizziness
- •
- sweating
- •
- flushing
- •
- high body temperature (hyperthermia)
- •
- tremors, stiff muscles, or muscle twitching
- •
- loss of coordination
- •
- seizures
- •
- nausea, vomiting, diarrhea
- •
-
Increased risk of bleeding. Taking vilazodone hydrochloride tablets with aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), warfarin or blood thinners may add to this risk. Tell your healthcare provider right away about any unusual bleeding or bruising.
- •
- Mania or hypomania (manic episodes) in people who have a history of bipolar disorder. Symptoms may include:
- •
- greatly increased energy
- •
- severe trouble sleeping
- •
- racing thoughts
- •
- reckless behavior
- •
- unusually grand ideas
- •
- excessive happiness or irritability
- •
- talking more or faster than usual
- •
- Discontinuation syndrome. Suddenly stopping vilazodone hydrochloride tablets may cause you to have serious side effects. Your healthcare provider may want to decrease your dose slowly. Symptoms may include:
- •
- nausea
- •
- sweating
- •
- changes in your mood
- •
- headache
- •
- irritability and agitation
- •
- tiredness
- •
- dizziness
- •
- problems sleeping
- •
- electric shock sensation (paresthesia)
- •
- hypomania
- •
- anxiety
- •
- ringing in your ears (tinnitus)
- •
- confusion
- •
- seizures
- •
-
Seizures (convulsions).
- •
-
Eye problems (angle-closure glaucoma): vilazodone hydrochloride tablets may cause a certain type of eye problem called angle-closure glaucoma. Call your healthcare provider if you have changes in your vision or eye pain.
- •
- Low sodium levels in your blood (hyponatremia). Low sodium levels in your blood may be serious and may cause death. Elderly people may be at greater risk for this. Signs and Symptoms of low sodium levels in your blood may include:
- •
- headache
- •
- difficulty concentrating
- •
- memory changes
- •
- confusion
- •
- weakness and unsteadiness on your feet which can lead to falls
In severe or more sudden cases, signs and symptoms include:
- •
- hallucinations (seeing or hearing things that are not real)
- •
- fainting
- •
- seizures
- •
- coma
- •
- respiratory arrest
- •
- death
- •
- Sexual problems (dysfunction). Taking selective serotonin reuptake inhibitors (SSRIs), including vilazodone hydrochloride tablets, may cause sexual problems.Symptoms in males may include:
- •
- delayed ejaculation or inability to have an ejaculation
- •
- problems getting or keeping an erection
- •
- decreased sex drive
Symptoms in females may include:
- •
- decreased sex drive
- •
- delayed orgasm or inability to have an orgasm
Talk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with vilazodone hydrochloride tablets. There may be treatments your healthcare provider can suggest.
The most common side effects of vilazodone hydrochloride tablets include diarrhea, nausea or vomiting, trouble sleeping.
These are not all the possible side effects of vilazodone hydrochloride tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store vilazodone hydrochloride tablets?- •
- Store vilazodone hydrochloride tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
-
Keep vilazodone hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of vilazodone hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use vilazodone hydrochloride tablets for a condition for which it was not prescribed. Do not give vilazodone hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them. You may ask your healthcare provider or pharmacist for information about vilazodone hydrochloride that is written for healthcare professionals.
What are the ingredients in vilazodone hydrochloride tablets?
Active ingredient: vilazodone hydrochloride
Inactive ingredients: colloidal silicon dioxide, FD&C Blue #1 Aluminum Lake (40 mg only), Ferric Oxide Red (10 mg only) and FD&C Yellow #6 Aluminum Lake (20 mg only), lactose monohydrate, macrogol (polyethylene glycol), magnesium stearate, microcrystalline cellulose, opadry II blue (for 40 mg), opadry II pink (for 10 mg) and opadry II orange (for 20 mg), polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
MSN Laboratories Private Limited
Telangana – 509 228,
INDIA
Distributed by:
Novadoz Pharmaceuticals LLC
Piscataway, NJ 08854-3714
Issued on: June 2023
Repackaged By: Preferred Pharmaceuticals Inc. - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
VILAZODONE HYDROCHLORIDE
vilazodone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68788-8734(NDC:72205-261) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VILAZODONE HYDROCHLORIDE (UNII: U8HTX2GK8J) (VILAZODONE - UNII:S239O2OOV3) VILAZODONE HYDROCHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TALC (UNII: 7SEV7J4R1U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE Score no score Shape OVAL (ellipse,biconvex) Size 11mm Flavor Imprint Code MV;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8734-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208228 09/16/2024 VILAZODONE HYDROCHLORIDE
vilazodone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68788-8735(NDC:72205-262) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VILAZODONE HYDROCHLORIDE (UNII: U8HTX2GK8J) (VILAZODONE - UNII:S239O2OOV3) VILAZODONE HYDROCHLORIDE 40 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score no score Shape OVAL (ellipse,biconvex) Size 14mm Flavor Imprint Code MV;16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8735-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208228 09/16/2024 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 REPACK(68788-8734, 68788-8735)