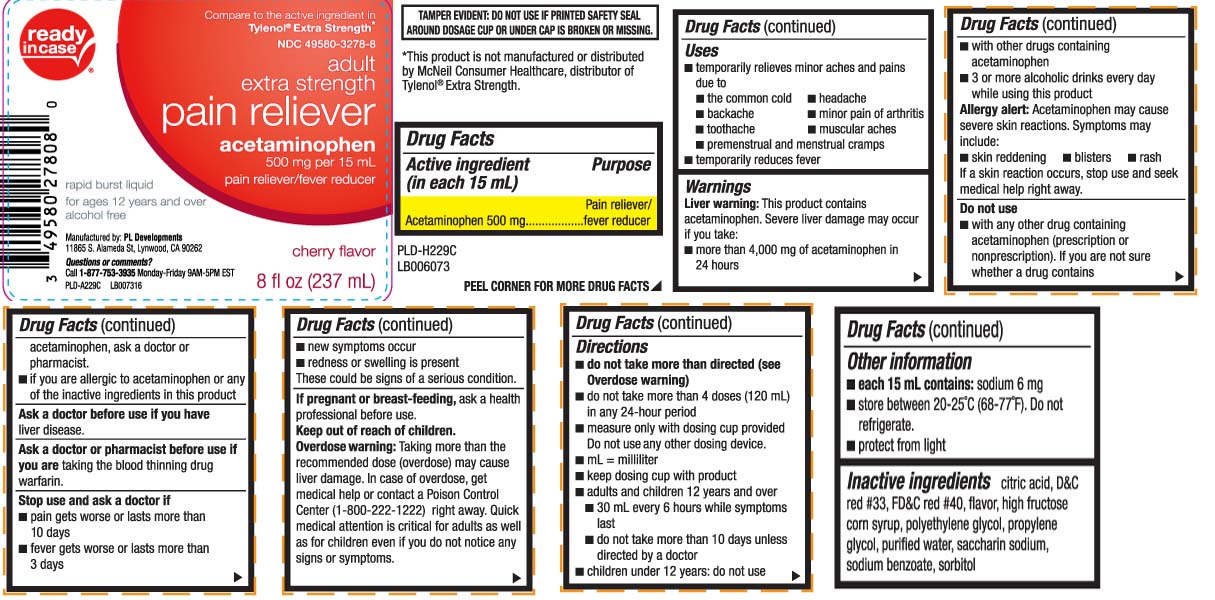
Label: PAIN RELIEVER EXTRA STRENGTH- acetaminophen liquid
- NDC Code(s): 49580-3278-8
- Packager: P & L Development, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 15 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks ever day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Stop use and ask a doctor if
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Directions
- do not take more than directed (see overdose warning)
- do not take more then 4 doses in any 24 hours period
- measure only with dosing cup provided. Do not use any other dosing device
- mL=milliliter
- keep dosing cup with product
- adults and children 12 years and over
- 30 mL every 6 hours while symptoms last
- do not take more than 10 days unless directed by a doctor
- children under 12 years: do not use
- Other information
- Inactive ingredients
-
Principal Display Panel
Compare to the active ingredient in Tylenol® Extra Strength*
adult
extra strength
pain reliever
acetaminophen 500 mg per 15 mL
pain reliever/fever reducer
rapid burst liquid
for ages 12 years and over
alcohol free
cherry flavor
FL OZ (mL)
Questions or comments?
Call 1-877-753-3935 Monday-Friday 9AM-5PM
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND DOSAGE CUP OR UNDER CAP IS BROKEN OR MISSING.
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Tylenol® Extra Strength.
Manufactured by: PL Developments
11865 S. Alameda St, Lynwood, CA 90262
- Product Label
-
INGREDIENTS AND APPEARANCE
PAIN RELIEVER EXTRA STRENGTH
acetaminophen liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49580-3278 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49580-3278-8 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/29/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 11/29/2019 Labeler - P & L Development, LLC (101896231)