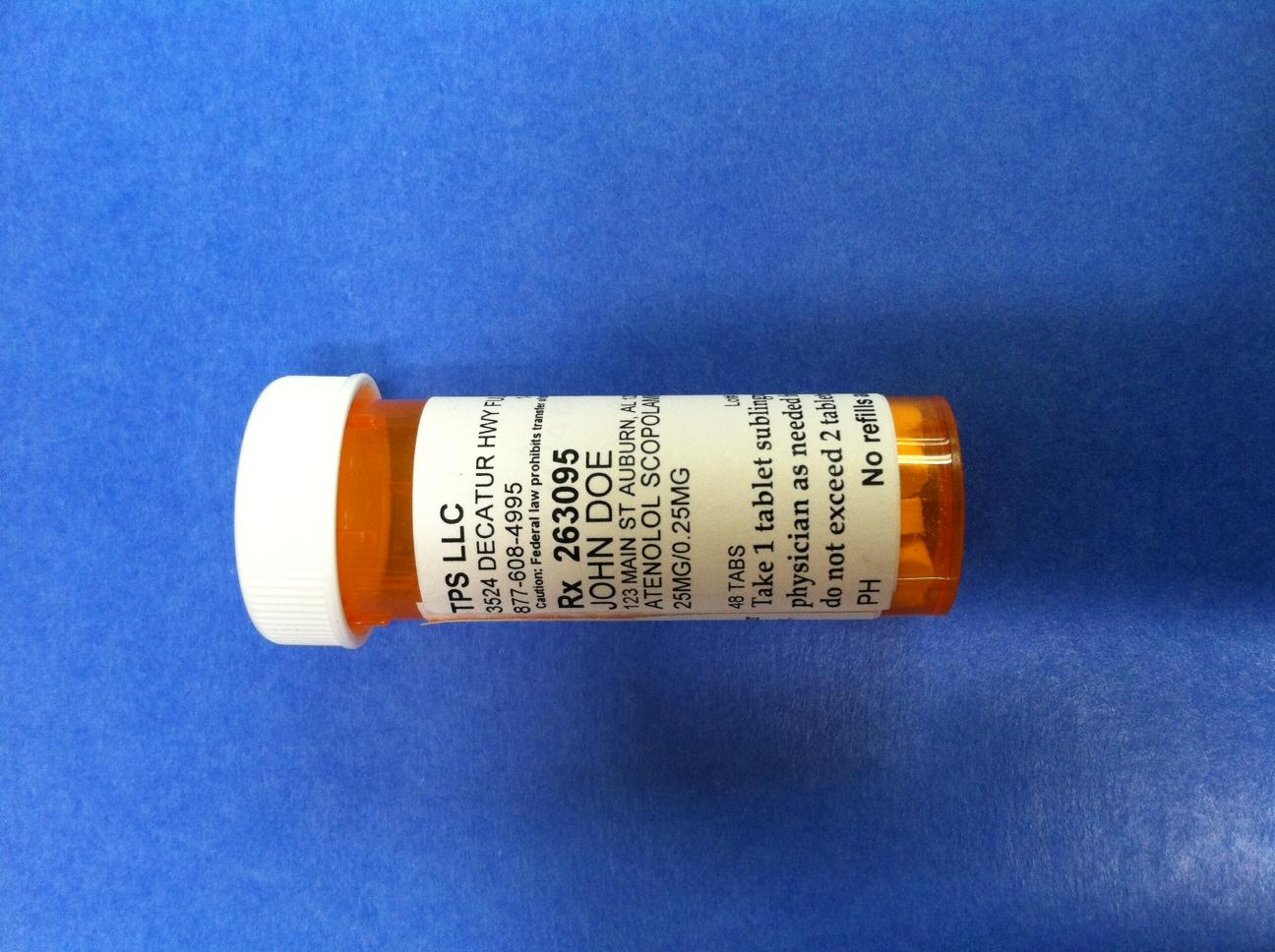
Label: ATENOLOL SCOPOLAMINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 69267-101-06, 69267-101-12, 69267-101-24, 69267-101-48 - Packager: TPS
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 14, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INSTRUCTIONS FOR USE
-
PRINCIPAL DISPLAY PANEL
TPS LLC
3524 DECATUR HWY FULTONDALE, AL 35068
877-608-4995 1-877-608-4995 BT9752747
Caution: Federal law prohibits transfer of this drug to any other person than patient for whom prescribed
Rx 263095 Jack Doe/Dr. Jane Doe MD
JOHN DOE
123 MAIN ST AUBURN, AL 12345
ATENOLOL SCOPOLAMINE TABLET TRITURATE
25 MG/0.25 MG
48 TABS Lot# Exp
Take 1 tablet sublingually or orally as directed by your physician as needed for symptoms of panic or anxiety.
Do not exceed 2 tablets per day
PH No refills authorized 10/8/2014
Pill bottle low res.jpg
-
INGREDIENTS AND APPEARANCE
ATENOLOL SCOPOLAMINE
atenolol scopolamine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69267-101 Route of Administration SUBLINGUAL, BUCCAL, TRANSMUCOSAL, ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATENOLOL (UNII: 50VV3VW0TI) (ATENOLOL - UNII:50VV3VW0TI) ATENOLOL 25 mg in 25.25 mg SCOPOLAMINE HYDROBROMIDE (UNII: 451IFR0GXB) (SCOPOLAMINE - UNII:DL48G20X8X) SCOPOLAMINE HYDROBROMIDE .25 mg in 25.25 mg Product Characteristics Color white Score no score Shape ROUND (The diameter depends on dies) Size 5mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69267-101-24 606 mg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2014 2 NDC:69267-101-06 151.5 mg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2014 3 NDC:69267-101-12 303 mg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2014 4 NDC:69267-101-48 1212 mg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2014 Labeler - TPS (044805267) Establishment Name Address ID/FEI Business Operations TPS 044805267 manufacture(69267-101)