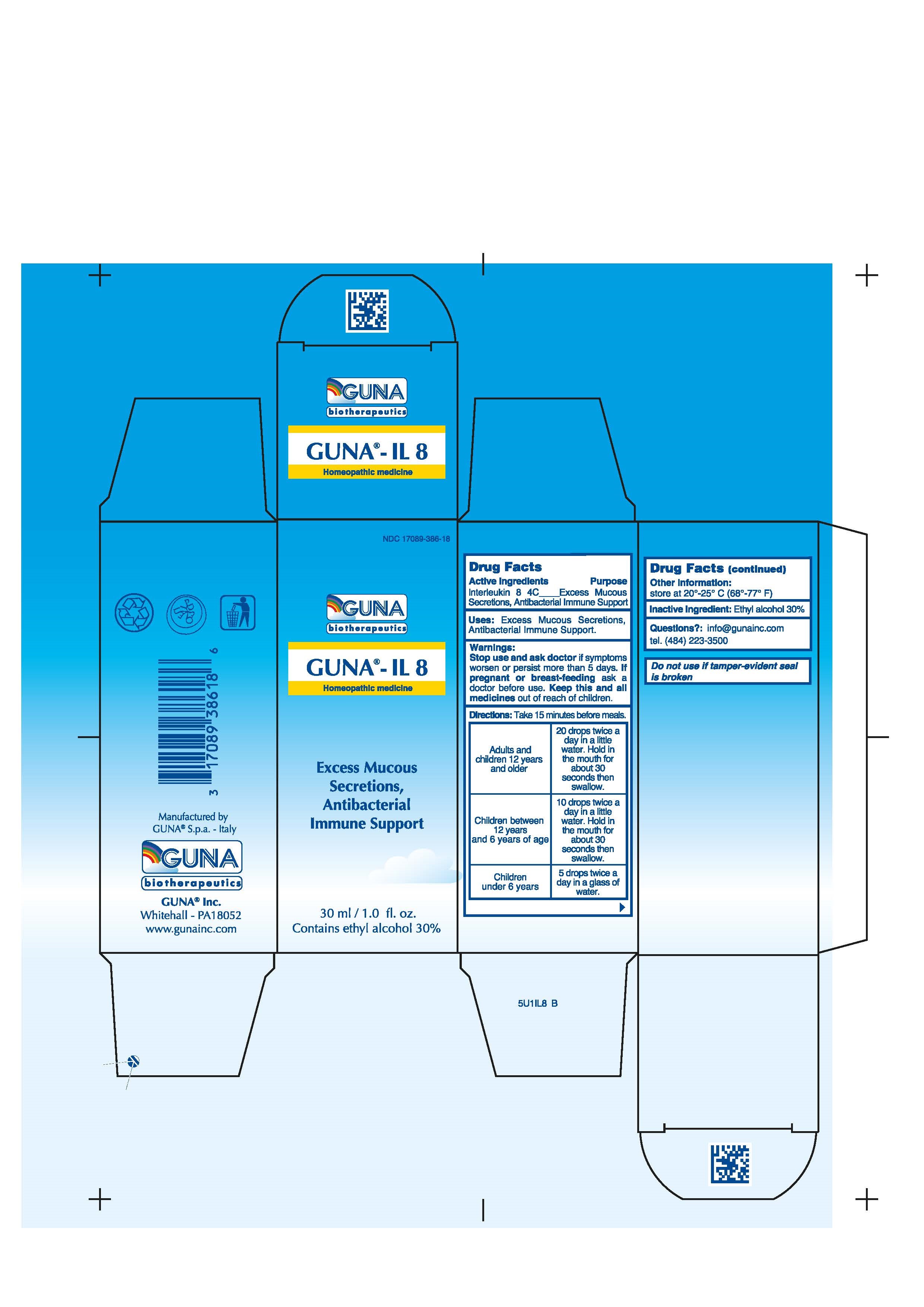
GUNA-IL 8- interleukin-8 solution/ drops
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
GUNA-IL 8
DIRECTIONS
Take 15 minutes before meals.
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.
| GUNA-IL 8
interleukin-8 solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guna spa | 338587646 | manufacture(17089-386) | |
Revised: 12/2018
Document Id: 7e9196de-3824-5731-e053-2a91aa0ad61f
Set id: 04abfa16-8c17-429a-904b-b1d9f5bddff1
Version: 2
Effective Time: 20181221
Guna spa