Label: FLURBIPROFEN SODIUM solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 42254-023-25 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 60758-910
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 13, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Flurbiprofen sodium ophthalmic solution, USP 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use.
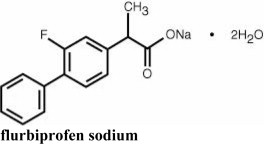
Chemical Name: Sodium (±)-2-(2-fluoro-4-biphenylyl)-propionate dihydrate.
Structural Formula:
Contains: Active: flurbiprofen sodium 0.03% (0.3mg/mL).
Preservative: thimerosal 0.005%. Inactives: citric acid; edetate disodium; polyvinyl alcohol 1.4%; potassium chloride; purified water; sodium chloride; and sodium citrate. May also contain hydrochloric acid and/or sodium hydroxide to adjust the pH. The pH of flurbiprofen sodium ophthalmic solution 0.03% is 6.0 to 7.0. It has an osmolality of 260 - 330 mOsm/kg.
-
CLINICAL PHARMACOLOGY
Flurbiprofen sodium is one of a series of phenylalkanoic acids that have shown analgesic, antipyretic, and anti-inflammatory activity in animal inflammatory diseases. Its mechanism of action is believed to be through inhibition of the cyclo-oxygenase enzyme that is essential in the biosynthesis of prostaglandins.
Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed on animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilatation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
Prostaglandins also appear to play a role in the miotic response produced during ocular surgery by constricting the iris sphincter independently of cholinergic mechanisms. In clinical studies, flurbiprofen sodium ophthalmic solution 0.03% has been shown to inhibit the miosis induced during the course of cataract surgery. Results from clinical studies indicate that flurbiprofen sodium has no significant effect upon intraocular pressure.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
With nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding due to interference with thrombocyte aggregation. There have been reports that flurbiprofen sodium ophthalmic solution 0.03% may cause increased bleeding of ocular tissues including hyphemas in conjunction with ocular surgery.
There exists the potential for cross-sensitivity to acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
-
PRECAUTIONS
General: Wound healing may be delayed with the use of flurbiprofen sodium ophthalmic solution 0.03%.
It is recommended that flurbiprofen sodium ophthalmic solution 0.03% be used with caution in surgical patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
Drug interactions: Interaction of flurbiprofen sodium ophthalmic solution 0.03% with other topical ophthalmic medications has not been fully investigated.
Although clinical studies with acetylcholine chloride and animal studies with acetylcholine chloride or carbachol revealed no interference, and there is no known pharmacological basis for an interaction, there have been reports that acetylcholine chloride and carbachol have been ineffective when used in patients treated with flurbiprofen sodium ophthalmic solution 0.03%.
Carcinogenesis, Mutagenesis, Impairment of fertility: Long-term studies in mice and/or rats have shown no evidence of carcinogenicity with flurbiprofen. Long-term mutagenicity studies in animals have not been performed.
Pregnancy category C. Flurbiprofen has been shown to be embryocidal, delay parturition, prolong gestation, reduce weight, and/or slightly retard growth of fetuses when given to rats in daily oral doses of 0.4 mg/kg (approximately 333 times the human daily topical dose) and above.
Nursing mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from flurbiprofen sodium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of flurbiprofen sodium ophthalmic solution 0.03%. Other adverse reactions reported with the use of flurbiprofen sodium ophthalmic solution 0.03% include: fibrosis, miosis, and mydriasis.
Increased bleeding tendency of ocular tissues in conjunction with ocular surgery has also been reported.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
-
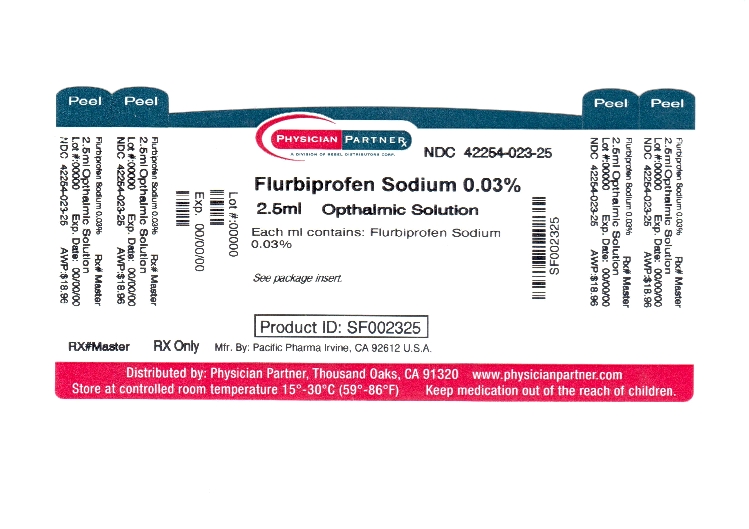
HOW SUPPLIED
Flurbiprofen sodium ophthalmic solution, USP is available for topical ophthalmic administration as a 0.03% sterile solution, and is supplied in a white opaque low density polyethylene bottle with a controlled dropper tip and a gray high impact polystyrene cap in the following size:
2.5 mL in 5 mL bottle - NDC 42254-023-25
Note: Store at 15°-25°C (59°-77°F).
Rx Only
Revised January 2004
© 2004 PACIFIC PHARMA
Irvine, CA 92612, U.S.A.
71587PY11P
Relabeled by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FLURBIPROFEN SODIUM
flurbiprofen sodium solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42254-023(NDC:60758-910) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength flurbiprofen sodium (UNII: Z5B97MU9K4) (flurbiprofen - UNII:5GRO578KLP) flurbiprofen sodium 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength thimerosal (UNII: 2225PI3MOV) citric acid monohydrate (UNII: 2968PHW8QP) edetate disodium (UNII: 7FLD91C86K) polyvinyl alcohol (UNII: 532B59J990) potassium chloride (UNII: 660YQ98I10) water (UNII: 059QF0KO0R) sodium chloride (UNII: 451W47IQ8X) sodium citrate (UNII: 1Q73Q2JULR) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42254-023-25 1 in 1 CARTON 1 2.5 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019404 05/29/1997 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK