Label: HYDROCORTISONE ACETATE suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 76413-132-12 - Packager: Central Texas Community Health Centers
- This is a repackaged label.
- Source NDC Code(s): 0574-7090
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 8, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
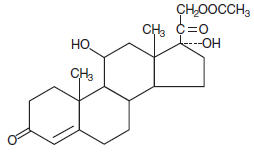
Each suppository for rectal administration contains 25 mg hydrocortisone acetate USP in a hydrogenated cocoglyceride base. Hydrocortisone acetate is a corticosteroid.
Chemically, hydrocortisone acetate is pregn-4-ene-3, 20 dione, 21- (acetyloxy)-11, 17-dihydroxy-, (11β)- with the following structural formula:
-
CLINICAL PHARMACOLOGY
In normal subjects, about 26 percent of hydrocortisone acetate is absorbed when the hydrocortisone acetate suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed surfaces.
Topical steroids are primarily effective because of their anti-inflammatory, antipruritic and vasoconstrictive action.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
Do not use unless adequate proctologic examination is made.
If irritation develops, the product should be discontinued and appropriate therapy instituted.
In the presence of an infection, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
No long-term studies in animals have been performed to evaluate the carcinogenic potential of corticosteroid suppositories.
PREGNANCY CATEGORY C
In laboratory animals, topical steroids have been associated with an increase in the incidence of fetal abnormalities when gestating females have been exposed to rather low dosage levels. There are no adequate and well-controlled studies in pregnant women.
It is not known whether this drug is excreted in human milk.
Until adequate studies in pregnant or lactating women have been conducted, this drug should be used during pregnancy or by nursing mothers only when clearly needed and when the potential benefits outweigh the potential risks.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
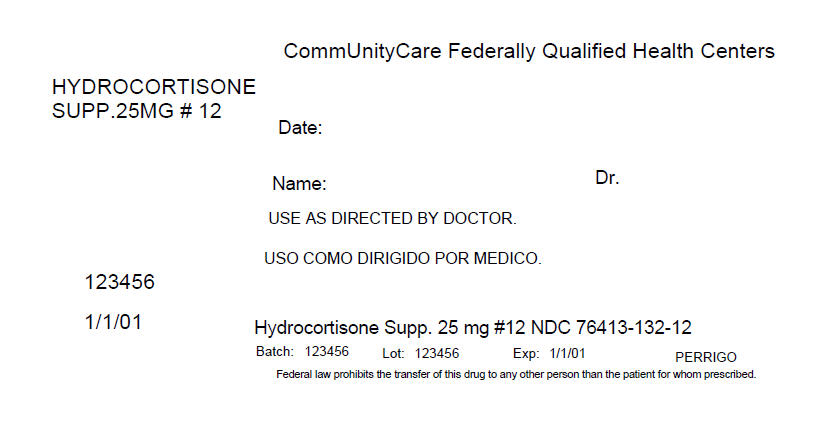
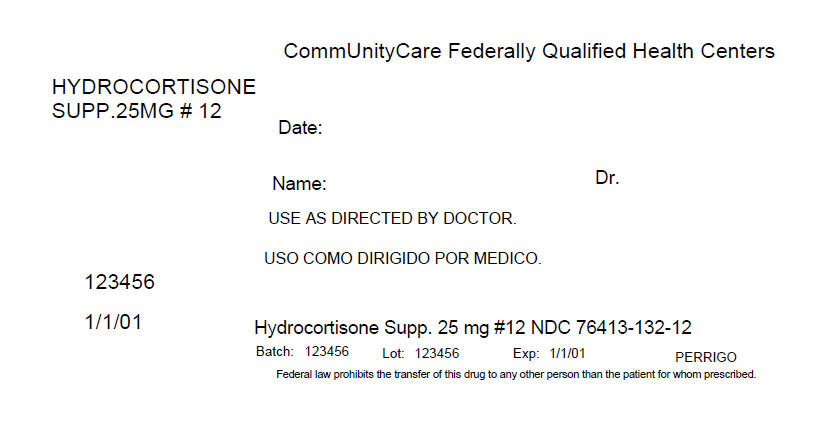
PRINCIPAL DISPLAY PANEL - 12 Packet Box Label
CommUnityCare Federally Qualified Health Centers
HYDROCORTISONE
SUPP.25MG # 12Date:
Name:
Dr.USE AS DIRECTED BY DOCTOR.
123456
1/1/01
Hydrocortisone Supp. 25 mg #12 NDC 76413-132-12
Batch: 123456
Lot: 123456
Exp: 1/1/01
PERRIGOFederal law prohibits the transfer of this drug to any other person than the patient for whom prescribed.
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE ACETATE
hydrocortisone acetate suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76413-132(NDC:0574-7090) Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 25 mg Product Characteristics Color WHITE Score no score Shape BULLET Size 35mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76413-132-12 12 in 1 BOX 07/01/1990 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 07/01/1990 Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Central Texas Community Health Centers 079674019 REPACK(76413-132) , RELABEL(76413-132)