I-DROPS ARTIFICIAL TEARS- hypromellose, naphazoline hydrochloride, polysorbate 80, zinc sulfate solution/ drops
GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
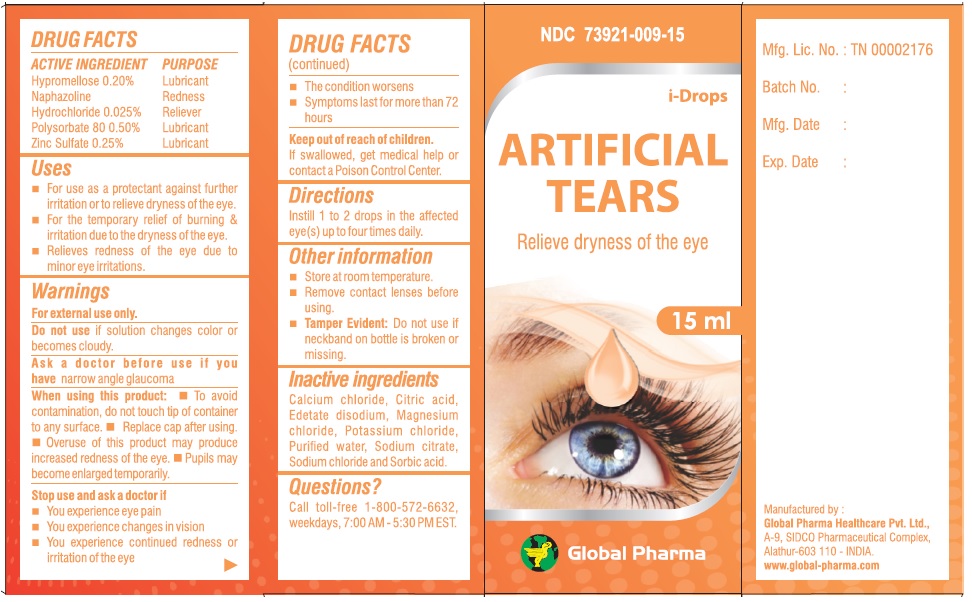
i-Drops ARTIFICIAL TEARS
ACTIVE INGREDIENT
Hypromellose 0.20%
Naphazoline Hydrochloride 0.025%
Polysorbate 80 0.50%
Zinc Sulfate 0.25%
Uses
• For use as a protectant against further irritation or to relieve dryness of the eye.
• For the temporary relief of burning & irritation due to the dryness of the eye.
• Relieves redness of the eye due to minor eye irritations.
Warnings
For external use only.
Do not use if solution changes color or becomes cloudy.
Ask a doctor before use if you have narrow angle glaucoma
When using this product: • To avoid contamination, do not touch tip of container to any surface. • Replace cap after using. • Overuse of this product may produce increased redness of the eye. • Pupils may become enlarged temporarily.
Stop use and ask a doctor if
• You experience eye pain
• You experience changes in vision
• You experience continued redness or irritation of the eye
• The condition worsens
• Symptoms last for more than 72 hours
Other information
• Store at room temperature.
• Remove contact lenses before using.
• Tamper Evident: Do not use if neckband on bottle is broken or missing.
Inactive ingredients
Calcium chloride, Citric acid, Edetate disodium, Magnesium chloride, Potassium chloride, Purified water, Sodium citrate, Sodium chloride and Sorbic acid.
| I-DROPS ARTIFICIAL TEARS
hypromellose, naphazoline hydrochloride, polysorbate 80, zinc sulfate solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED (860186917) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED | 860186917 | manufacture(73921-009) | |