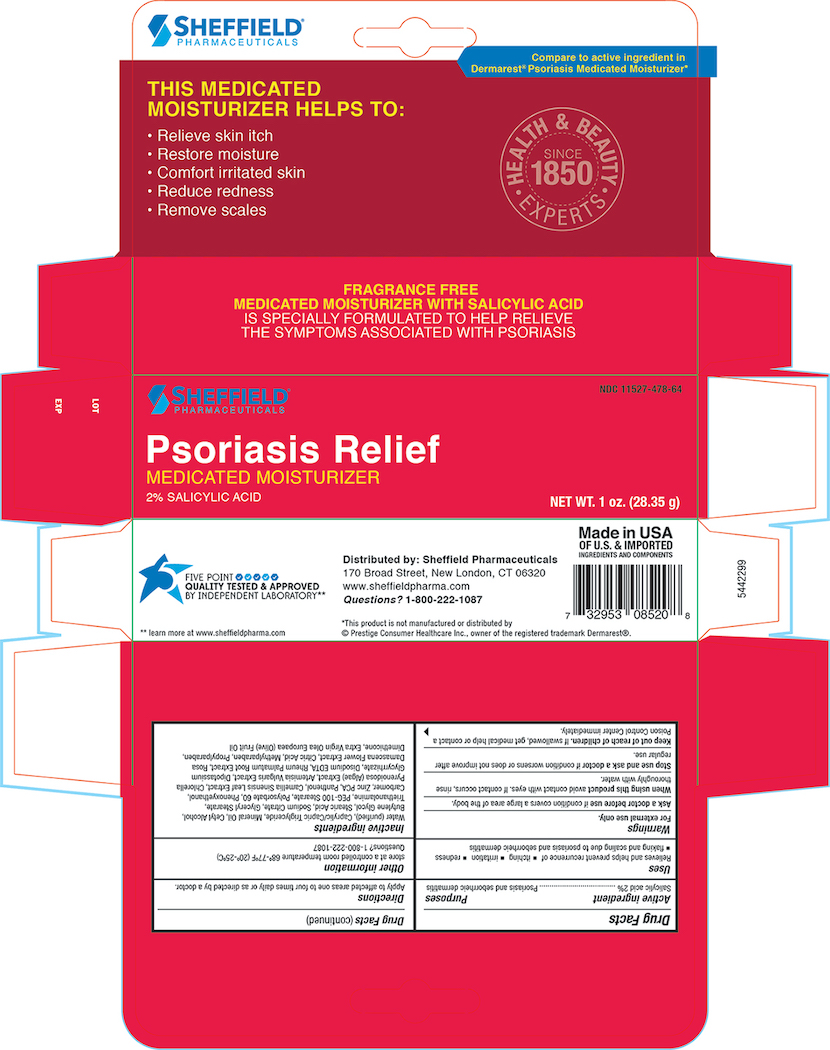
Label: DR. SHEFFIELD PSORIASIS MEDICATED MOISTURIZER- psoriasis medicated moisturizer lotion
- NDC Code(s): 11527-478-64
- Packager: Sheffield Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purposes
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
Purifed Water, Caprylic/Capric Triglyceride, Mineral Oil, Cetyl Alcohol, Butylene Glycol, Stearic Acid, Sodium Citrate, Glyceral Stearate, Triethanolamine, PEG-100 Sterate, Polysorbate 60 , Phenoxyethanol, Carbomer, Zinc PCA, Panthenol, Camellia Oleifera leaf extract , Algea Extract, Artemisia Vulgaris Extract, Dipotassium Glycyrrhizate, Disodium EDTA, Rheum Palmatum Extract, Rosa Damascena Extract, Citric Acid, Methylparaben, Propylparaben, Dimethicone, Extra Virign Olea Europaea( Olive) Fruit oil
- Principal Display Panel - Carton 1.0 oz
- Principal Display Panel 1 oz. - Tube
-
INGREDIENTS AND APPEARANCE
DR. SHEFFIELD PSORIASIS MEDICATED MOISTURIZER
psoriasis medicated moisturizer lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11527-478 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) TROLAMINE (UNII: 9O3K93S3TK) PEG-100 STEARATE (UNII: YD01N1999R) POLYSORBATE 60 (UNII: CAL22UVI4M) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOMER 934 (UNII: Z135WT9208) ZINC PIDOLATE (UNII: C32PQ86DH4) PANTHENOL (UNII: WV9CM0O67Z) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) ARTEMISIA VULGARIS ROOT (UNII: 32MP823R8S) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) RHEUM PALMATUM ROOT (UNII: G025DAL7CE) ROSA DAMASCENA FLOWER (UNII: JWB78P295A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIMETHICONE (UNII: 92RU3N3Y1O) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11527-478-64 1 in 1 CARTON 09/01/2013 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 09/01/2013 Labeler - Sheffield Pharmaceuticals LLC (151177797) Registrant - Sheffield Pharmaceuticals LLC (151177797) Establishment Name Address ID/FEI Business Operations Sheffield Pharmaceuticals LLC 151177797 manufacture(11527-478)