Label: MEMBRANEBLUE- trypan blue injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 68803-672-05 - Packager: Dutch Ophthalmic Research Center (International) B.V.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 15, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
MembraneBlue™ 0.15% (trypan blue ophthalmic solution). These highlights do not include all the information needed to use MembraneBlue™ 0.15% safely and effectively. See full prescribing information for MembraneBlue™ 0.15%. MembraneBlue™ 0.15% (trypan blue ophthalmic solution) Initial U.S. Approval: 2004
INDICATIONS AND USAGE
HIGHLIGHTS OF PRESCRIBING INFORMATION (1)
These highlights do not include all the information needed to use MembraneBlue™ 0.15% safely and effectively. See full prescribing information for MembraneBlue™ 0.15%. MembraneBlue™ 0.15% (trypan blue ophthalmic solution) Initial U.S. Approval: 2004 (1)
(1)
- For use as an aid in ophthalmic posterior surgery;
- Facilitating removal of epiretinal tissue.
DOSAGE AND ADMINISTRATION
- Prior to injection of MembraneBlue™ 0.15% perform a 'fluid-air exchange'; Carefully apply MembraneBlue™ 0.15% to epiretinal membranes using a blunt cannula; Remove all excess dye; Or
- Inject MembraneBlue™ 0.15% directly in a BSS filled - vitreous cavity; Wait 30 seconds; Remove all excess dye.
DOSAGE FORMS AND STRENGTHS
MembraneBlue™ 0.15% (trypan blue ophthalmic solution) in a volume of 0.5 mL. (3)
CONTRAINDICATIONS
Insertion of a non-hydrated (dry state), hydrophilic acrylic intraocular lens (IOL). (4)
WARNINGS AND PRECAUTIONS
- Excessive staining: Excess MembraneBlue™ 0.15% should be removed from the eye immediately after staining.
- Priming of the syringe: make sure the plunger moves smoothly before use: first retract the plunger or twist the plunger in a clockwise motion before injecting the fluid.
ADVERSE REACTIONS
- Discoloration of high water content hydrogen intraocular lenses;
- Inadvertent staining of the posterior lens capsule and vitreous face;
To report suspected adverse reactions contact Dutch Ophthalmic, USA at 1-800-75-DUTCH or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Trypan blue should not be given to pregnant women. (7)
Revised: 11/2009
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
MembraneBlue 0.15% - Indications & Usage Section
MembraneBlue 0.15% - Dosage & administration section
MembraneBlue 0.15% - Dosage forms & strengths section.
MembraneBlue 0.15% - Contraindications section.
MembraneBlue 0.15% - Warnings and precautions section
MembraneBlue 0.15% - Adverse reactions section.
MembraneBlue 0.15% - Use in specific populations section
MembraneBlue 0.15% - Pregnancy section.
MembraneBlue 0.15% - Nursing mothers section
MembraneBlue 0.15% - Pediatric use section.
MembraneBlue 0.15% - Geriatric use.
MembraneBlue 0.15% - Description.
MembraneBlue 0.15% - Clinical Pharmacology Section.
MembraneBlue 0.15% - Mechanism of action section.
MembraneBlue 0.15% - Nonclinical toxicology section.
MembraneBlue 0.15% - How supplied section.
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- MembraneBlue 0.15% - Indications & Usage Section
-
MembraneBlue 0.15% - Dosage & administration section
Make sure the plunger moves smoothly before use. Prime the syringe prior to use by retracting the plunger before injecting the fluid. Alternatively, twist the plunger into the stopper in a clockwise motion until tight. Once tight, continue turning the plunger in a clockwise motion until the stopper rotates freely within the syringe, two or three rotations. The syringe is now primed and suitable for injection.
Before injection of MembraneBlue™ 0.15% perform a 'fluid-air exchange', i.e. filling the entire vitreous cavity with air, to prevent aqeous dilution of MembraneBlue™ 0.15%. MembraneBlue™ 0.15% is carefully applied to the retinal membrane using a blunt cannula attached to the MembraneBlue™ 0.15% syringe, without allowing the cannula to contact or damage the retina. Sufficient staining is expected on contact with the membrane. All excess dye should be removed from the vitreous cavity before performing an air-fluid exchange, to prevent unnecessary spreading of the dye.
MembraneBlue™ 0.15% can also be injected directly in a BSS filled vitreous cavity (instead of injecting under air). Clinical use demonstrated that, after complete vitreous and posterior hyaloid removal, sufficient staining is achieved after 30 seconds of application under BSS.
MembraneBlue™ 0.15% is intended to be applied directly on the areas where membranes could be present, staining any portion of the membrane which comes in contact with the dye. The dye does not penetrate the membrane.
- MembraneBlue 0.15% - Dosage forms & strengths section.
- MembraneBlue 0.15% - Contraindications section.
- MembraneBlue 0.15% - Warnings and precautions section
-
MembraneBlue 0.15% - Adverse reactions section.
Adverse reactions reported following use of MembraneBlue™ 0.15% include discoloration of high water content hydrogen intraocular lenses (see Contraindications) and inadvertent staining of the posterior lens capsule and vitreous face. Staining of the posterior lens capsule or staining of the vitreous face is generally self limited, lasting up to one week.
-
MembraneBlue 0.15% - Use in specific populations section
MembraneBlue 0.15% - Pregnancy section.
Teratogenic Effects: Pregnancy Category C. Trypan blue is teratogenic in rats, mice, rabbits, hamsters, dogs, guinea pigs, pigs, and chickens. The majority of teratogenicity studies performed involve intravenous, intraperitoneal, or subcutaneous administration in the rat. The teratogenic dose is 50 mg/kg as a single dose or 25 mg/kg/day during embryogenesis in the rat. These doses are approximately 4,000- and 2,000-fold the maximum recommended human dose of 0.75 mg per injection based on a 60 kg person, assuming that the whole dose is completely absorved. Characteristic anomalies included neural tube, cardiovascular, vertebral, tail, and eye defects. Trypan blue also caused an increase in post-implantation mortality, and decreased fetal weight. In the monkey, trypan blue caused abortions with single or two daily doses of 50 mg/kg between the 20th to 25th days of pregnancy, but no apparent increase in birth defects (approximately 4,000-fold maximum recommended human dose of 0.75 mg per injection, assuming total absorption). There are no adequate and well-controlled studies in pregnant women. Trypan blue should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
MembraneBlue 0.15% - Nursing mothers section
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when trypan blue is administered to a nursing woman.
-
MembraneBlue 0.15% - Description.
MembraneBlue™ 0.15% (trypan blue ophthalmic solution) is a sterile solution of trypan blue (an acid di-azo group dye). MembraneBlue™ 0.15% selectively stains epiretinal membranes during ophthalmic surgical vitrectomy procedures.
Each mL of MembraneBlue™ 0.15% contains: 1.5 mg trypan blue; 1.9 mg sodium mono-hydrogen orthophosphate (Na 2HPO 4•2H 2O); 0.3 mg sodium di-hydrogen orthophosphate (NaH 2PO 4•2H 2O); 8.2 mg sodium chloride (NaCl); and water for injection. The pH is 7.3 - 7.6. The osmolality is 257 - 314 mOsm/kg.
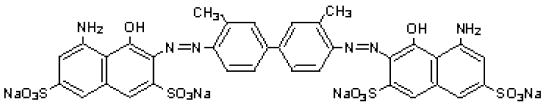
The drug substance trypan blue has the chemical name 3,3'-[(3,3'-dimethyl-4,4'-biphenylylene) bis (azo)] bis (5-amino-4-hydroxy-2,7-naphthalenedisulfonic acid) tetra sodium salt, a molecular weight of 960.8, a molecular formula of C 34H 24N 6Na 4O 14S 4, and has the following chemical structure:
- MembraneBlue 0.15% - Clinical Pharmacology Section.
-
MembraneBlue 0.15% - Nonclinical toxicology section.
Trypan blue is carcinogenic in rats. Wister/Lewis rats developed lymphomas after receiving subcutaneous injections of 1% trypan blue dosed at 50 mg/kg every other week for 52 weeks (total dose approximately 100,000-fold the maximum recommended human dose of 0.75 mg per injection in a 60 kg person,assuming total absorption).
- MembraneBlue 0.15% - How supplied section.
- MembraneBlue 0.15% - Storage and handling section.
-
SPL UNCLASSIFIED SECTION
Rx ONLY
Manufactured by
D.O.R.C. international b.v.
Scheijdelveweg 2
3214 VN Zuidland
The Netherlands
Distributed in the United States by
Dutch Ophthalmic, USA
10, Continental Drive, Bldg 1
Exeter, NH 03833, USA
Phone: 800-75-DUTCH or 603-778-6929
US Patents 6,696,430 and 6,372,449
Copyright ©, 2009 Dutch Ophthalmic Research Center
- MembraneBlue 0.15% - Package label.Principal display panel
-
INGREDIENTS AND APPEARANCE
MEMBRANEBLUE
trypan blue injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68803-672 Route of Administration OPHTHALMIC, INTRAOCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRYPAN BLUE (UNII: I2ZWO3LS3M) (TRYPAN BLUE FREE ACID - UNII:768N7QO4KH) TRYPAN BLUE 0.75 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 0.15 mg in 0.5 mL WATER (UNII: 059QF0KO0R) 0.5 mL in 0.5 mL SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) 0.95 mg in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.1 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68803-672-05 5 in 1 CARTON 02/20/2009 1 1 in 1 POUCH 1 0.5 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022278 02/20/2009 Labeler - Dutch Ophthalmic Research Center (International) B.V. (407522184) Registrant - Dutch Ophthalmic Research Center (International) B.V. (407522184) Establishment Name Address ID/FEI Business Operations Pharmpur GmbH 340805167 manufacture(68803-672)




