Label: ACETAMINOPHEN tablet, extended release
-
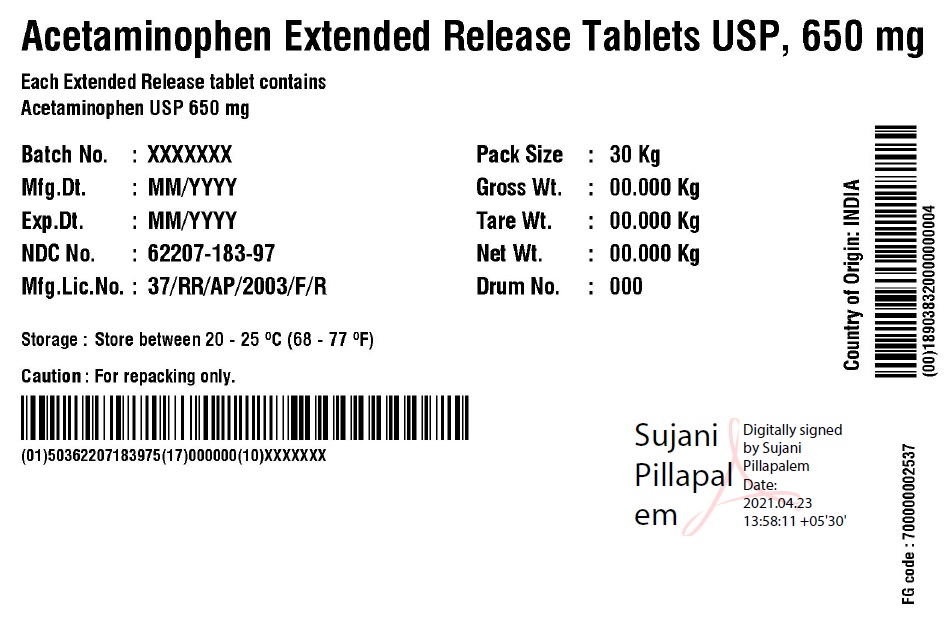
NDC Code(s):
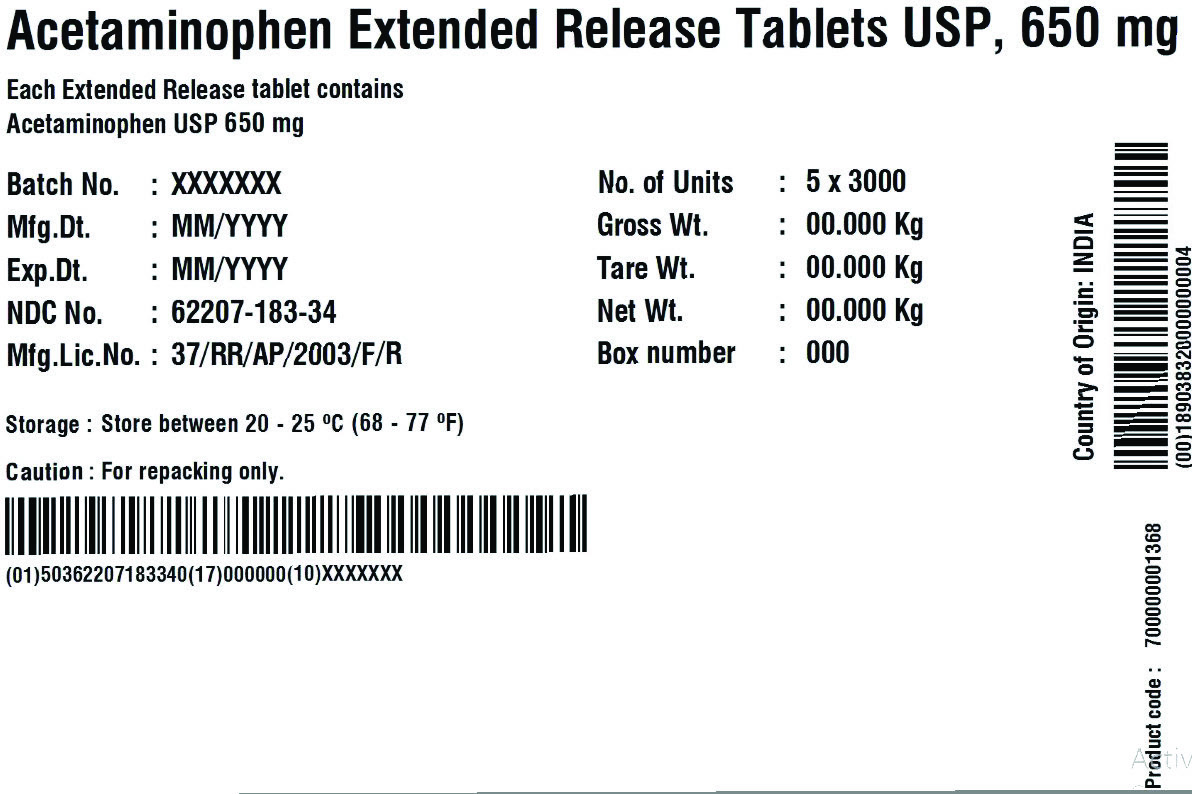
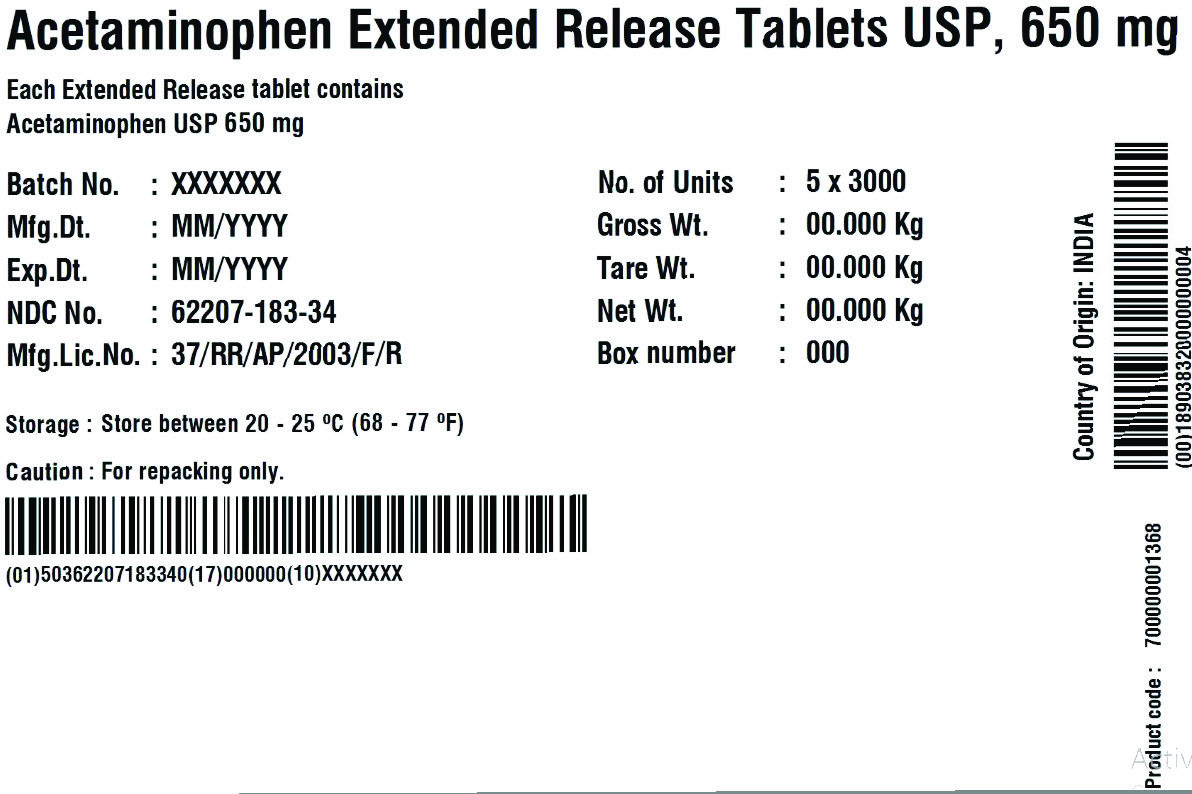
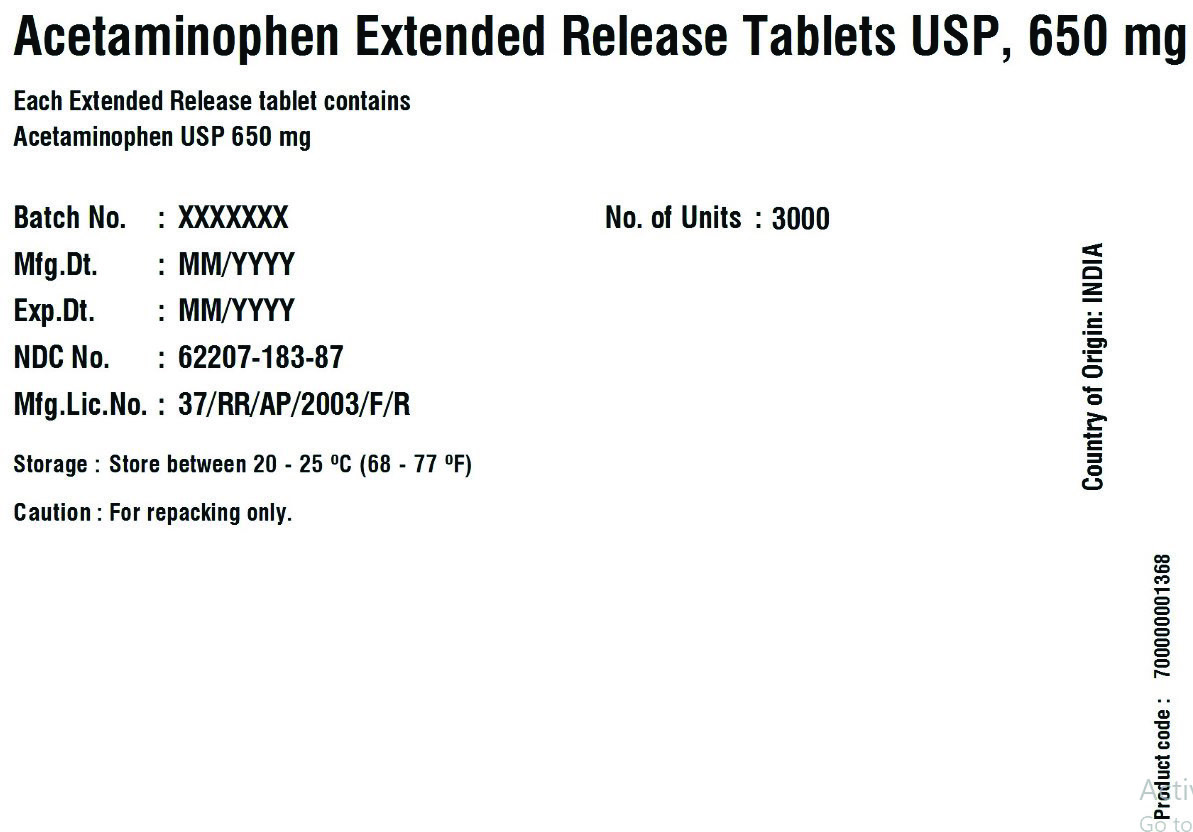
62207-183-34,
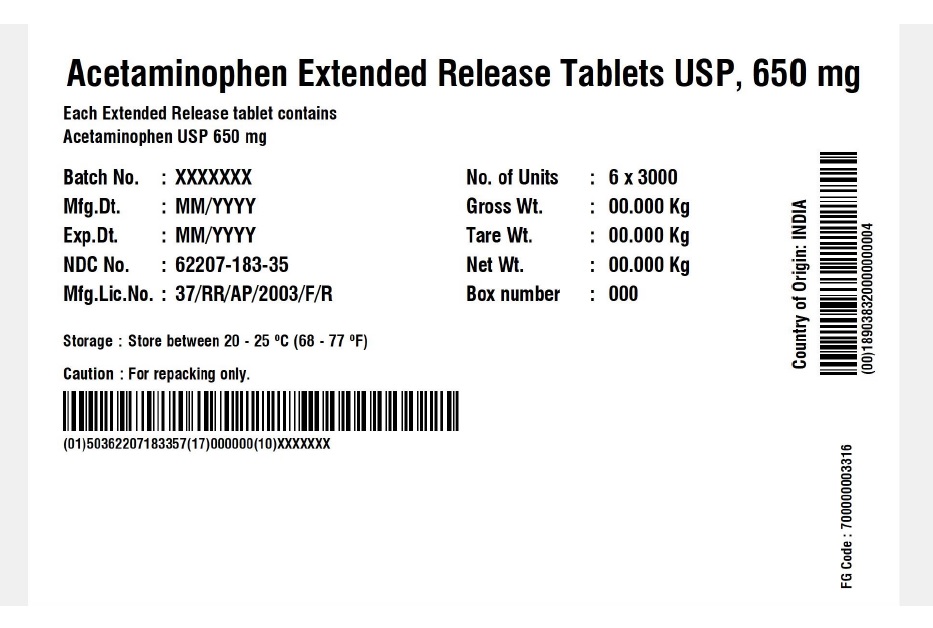
62207-183-35,
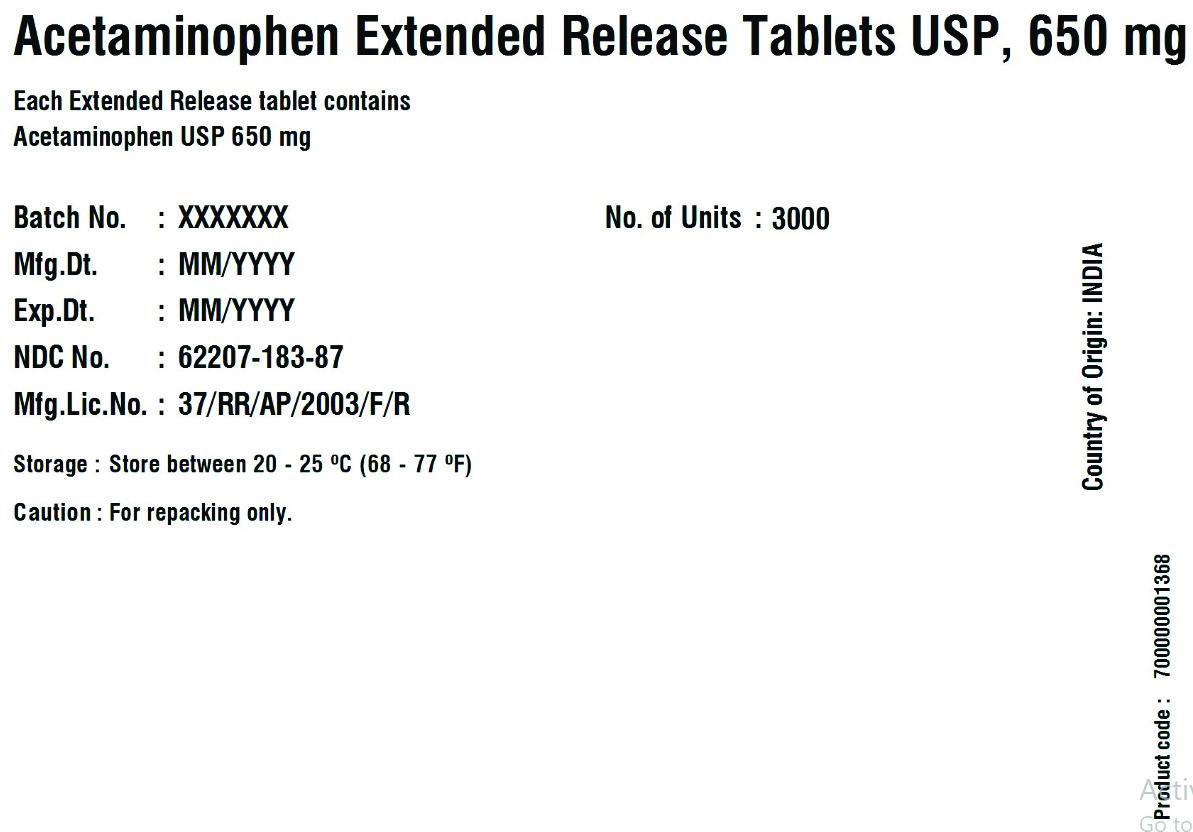
62207-183-49,
62207-183-51, view more62207-183-87, 62207-183-97, 62207-188-49, 62207-188-51
- Packager: Granules India Limited
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 4, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
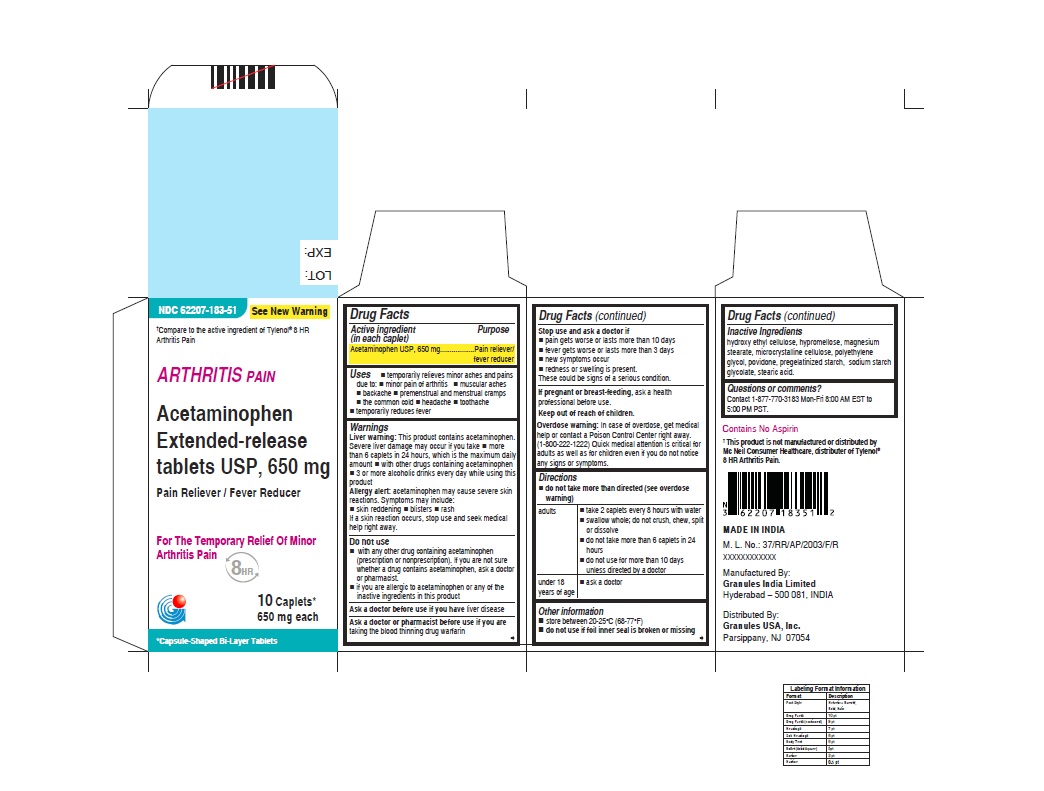
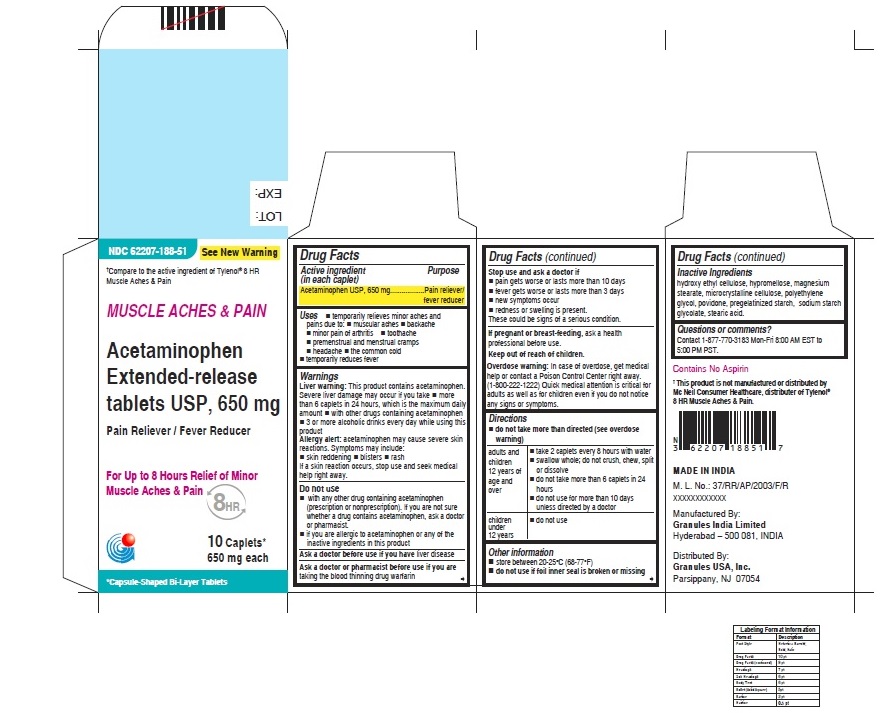
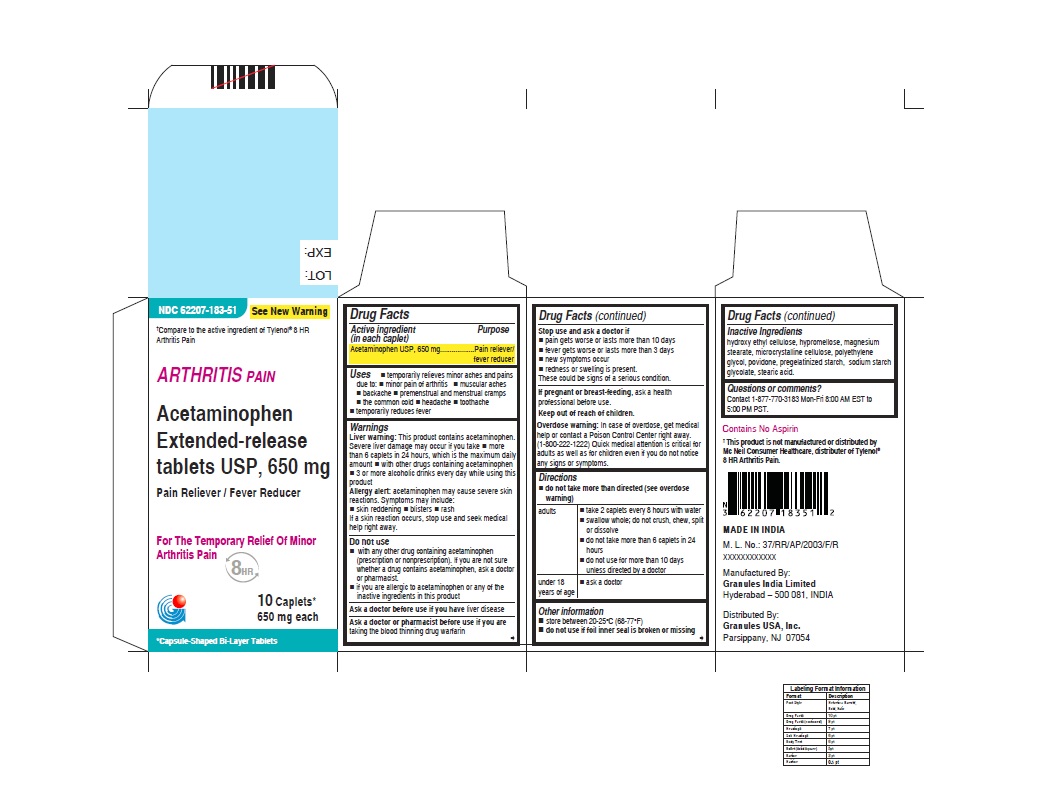
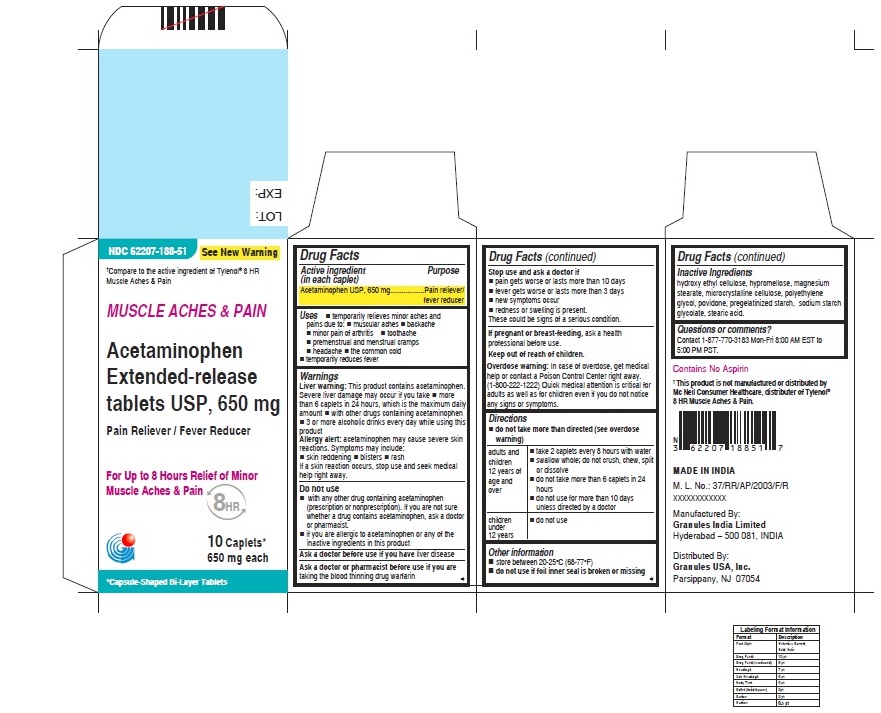
- ACTIVE INGREDIENT (IN EACH CAPLET)
- PURPOSE
-
USES
For Arthritis Pain label
• temporarily relieves minor aches and pains due to:
• minor pain of arthritis
• muscular aches
• backache
• premenstrual and menstrual cramps
• the common cold
• headache
• toothache
• temporarily reduces fever
For Muscle Aches & Pain label
• temporarily relieves minor aches and pains due to:
• muscular aches
• backache
• minor pain of arthritis
• toothache
• premenstrual and menstrual cramps
• headache
• the common cold
• temporarily reduces fever
-
WARNINGS
Liver warning: This product contains acetaminophen. Severe Liver damage may occur if you take
• more than 6 caplets in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks everyday while using this productAllergy alert: acetaminophen may cause severe skin reactions
Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away - Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
-
DIRECTIONS
For Arthritis Pain Label
• do not take more than directed (see overdose warning)
adults • take 2 caplets every 8 hours with water
• swallow whole; do not crush, chew, split or dissolve
• do not take more than 6 caplets in 24 hours
• do not use for more than 10 days unless directed by a doctorunder 18 years
of age• ask a doctor
For Muscle Ache and Pain label
• do not take more than directed (see overdose warning)
adults and children
12 years of age and over• take 2 caplets every 8 hours with water
• swallow whole; do not crush, chew, split or dissolve
• do not take more than 6 caplets in 24 hours
• do not use for more than 10 days unless directed by a doctorchildren under
12 years• do not use - OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS ?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-188 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-188-51 1 in 1 CARTON 04/18/2019 1 10 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:62207-188-49 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211544 04/18/2019 ACETAMINOPHEN
acetaminophen tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-183 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-183-51 1 in 1 CARTON 04/18/2019 1 10 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:62207-183-34 1 in 1 BOX 06/30/2019 2 5 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:62207-183-87 1 in 1 BOX 06/30/2019 3 1 in 1 POUCH; Type 0: Not a Combination Product 4 NDC:62207-183-49 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2019 5 NDC:62207-183-97 40000 in 1 DRUM; Type 0: Not a Combination Product 03/25/2021 6 NDC:62207-183-35 6 in 1 BOX 05/04/2024 6 3000 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211544 04/18/2019 Labeler - Granules India Limited (915000087) Registrant - Granules India Limited (915000087)