FLUORIDE - fluoride tablet, chewable
Mayne Pharma Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
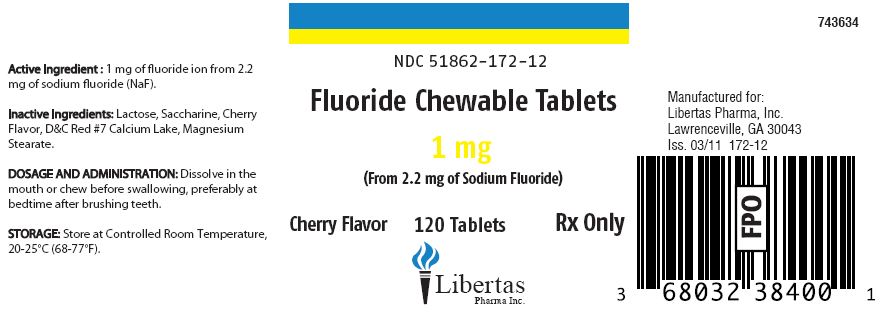
Fluoride Chewable Tablets 1 mg
Active Ingredient: 1 mg of fluoride ion from 2.2 mg of sodium fluoride (NaF).
Inactive Ingredients: Lactose, Saccharine, Cherry Flavor, D&C Red #7 Calcium Lake, Magnesium Stearate.
DOSAGE AND ADMINISTRATION:
Dissolve in the mouth or chew before swallowing, preferably at bedtime after brushing teeth.
| FLUORIDE
fluoride tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Mayne Pharma Inc (867220261) |
Revised: 7/2017
Document Id: 28d952e7-7caa-492f-a1f5-7a39098da55e
Set id: 00e1f644-1538-4054-b112-227e7254317e
Version: 2
Effective Time: 20170726
Mayne Pharma Inc