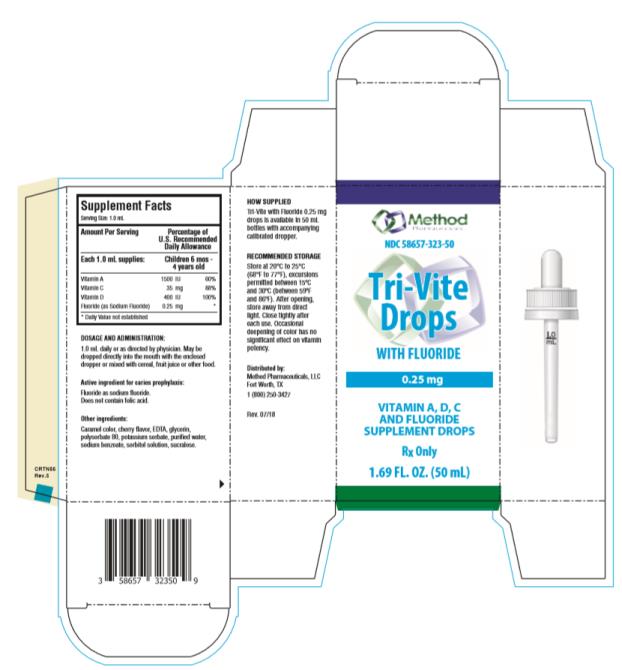
Label: TRI-VITE DROPS WITH FLUORIDE- ascorbic acid, sodium fluoride, vitamin a and vitamin d solution
- NDC Code(s): 58657-323-50
- Packager: Method Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Supplement Facts
Serving Size: 1.0 mL
Amount Per Serving Percentage of
U.S. Recommended
Daily AllowanceEach 1.0 mL supplies: Children 6 mos - 4 years old Vitamin A 1500 IU 60% Vitamin C 35 mg 88% Vitamin D 400 IU 100% Fluoride (as Sodium Fluoride) 0.25 mg * * Daily Value not established
See INDICATIONS AND USAGE section for use by children 6 months to 6 years of age. This product does not contain the essential vitamin folic acid.
Active ingredient for caries prophylaxis: Fluoride as sodium fluoride. Does not contain folic acid.
Other ingredients: caramel color, cherry flavor, EDTA, glycerin, polysorbate 80, potassium sorbate, purified water, sodium benzoate, sorbitol solution, sucralose.
-
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries. Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal, fluorapatite.
The reaction may be expressed by the equation:
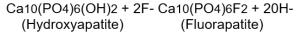
Three stages of fluoride deposition in tooth enamel can be distinguished:
1. Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed.
2. After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted.
3. After eruption, the surface enamel acquires fluoride from water, food, supplementary fluoride and smaller amounts from saliva.
-
INDICATIONS AND USAGE
Supplementation of the diet with vitamins A, C and D. Tri-Vite Drops with Fluoride 0.25 mg also provides fluoride for caries prophylaxis.
The American Academy of Pediatrics recommends that children up to age 16, in areas where drinking water contains less than optimal levels of fluoride, receive daily fluoride supplementation. The American Academy of Pediatrics recommend that infants and young children 6 months to 3 years of age, in areas where the drinking water contains less than 0.3 ppm of fluoride, and children 3-6 years of age, in areas where the drinking water contains 0.3 through 0.6 ppm of fluoride, receive 0.25 mg of supplemental fluoride daily which is provided in a dose of 1 mL of Tri-Vite Drops with Fluoride 0.25 mg (See Dosage and Administration).
Tri-Vite Drops with Fluoride 0.25 mg supply significant amounts of vitamins A, C and D to supplement the diet, and to help assure that nutritional deficiencies of these vitamins will not develop. Thus, in a single easy-to-use preparation, children obtain essential vitamins and fluoride.
- WARNINGS
-
PRECAUTIONS
The suggested dose should not be exceeded since dental fluorosis may result from continued ingestion of large amounts of fluoride.
When prescribing vitamin fluoride products:
1. Determine the fluoride content of the drinking water.
2. Make sure the child is not receiving significant amounts of fluoride from other medications and swallowed toothpaste.
3. Periodically check to make sure that the child does not develop significant dental fluorosis.
Tri-Vite Drops with Fluoride 0.25 mg should be dispensed in the original plastic container, since contact with glass leads to instability and precipitation. (The amount of sodium fluoride in the 50 mL size is well below the maximum to be dispensed at one time according to recommendations of the American Dental Association.)
Important Considerations When Using Dosage Schedule:
• If fluoride level is unknown, drinking water should be tested for fluoride content before supplements are prescribed. For testing of fluoride content, contact the local or state health department.
• All sources of fluoride should be evaluated with a thorough fluoride history. Patient exposure to multiple water sources can make proper prescribing complex.
• Ingestion of higher than recommended levels of fluoride by children has been associated with an increase in mild dental fluorosis in developing, unerupted teeth.
• Fluoride supplements require long-term compliance on a daily basis.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- RECOMMENDED STORAGE
-
REFERENCES
1. Brudevoid F, McCann HG: Fluoride and caries control - Mechanism of action, in Nizel AE (ed): The Science of Nutrition and its Application in Clinical Dentistry. Philadelphia, WB Saunders Co, 1966, pp 331-347.
2. American Academy of Pediatrics Committee on Nutrition: Fluoride supplementation, Pediatrics 1986; 77:758.
3. American Dental Association Council on Dental Therapeutics: Accepted Dental Therapeutics, ed 38, Chicago, 1979, p321.
4. Hennon DK, Stookey GK, Muhler JC: The clinical anticariogenic effectiveness of supplementary fluoride-vitamin preparations - Results at the end of three years. J Dent Children 1966; 33 January: 3-12.
5. Hennon DK, Stookey GK, Muhler JC: The clinical anticariogenic effectiveness of supplementary fluoride-vitamin preparations - Results at the end of four years. J Dent Children 1967; 34 November; 439- 443.
6. Hennon DK, Stookey GK, Muhler JC: The clinical anticariogenic effectiveness of supplementary fluoride-vitamin preparations - Results at the end of five and a half years. Phar and Ther in Dent 1970; 1:1.
7. Hennon DK, Stookey GK, Beiswanger BB: Fluoride-vitamin supplements: Effects on dental caries and fluorosis when used in areas with suboptimum fluoride in the water supply. J Am Dent Assoc 1977; 95-965
Distributed by:
Method Pharmaceuticals, LLC
Fort Worth, TX 76118
1-877-250-3427
Rev. 07/2018 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRI-VITE DROPS WITH FLUORIDE
ascorbic acid, sodium fluoride, vitamin a and vitamin d solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58657-323 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1500 [iU] in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 35 mg in 1 mL VITAMIN D (UNII: 9VU1KI44GP) (VITAMIN D - UNII:9VU1KI44GP) VITAMIN D 400 [iU] in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETIC ACID (UNII: 9G34HU7RV0) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color brown (caramel color) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58657-323-50 1 in 1 CARTON 07/25/2018 1 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/25/2018 Labeler - Method Pharmaceuticals, LLC (060216698)