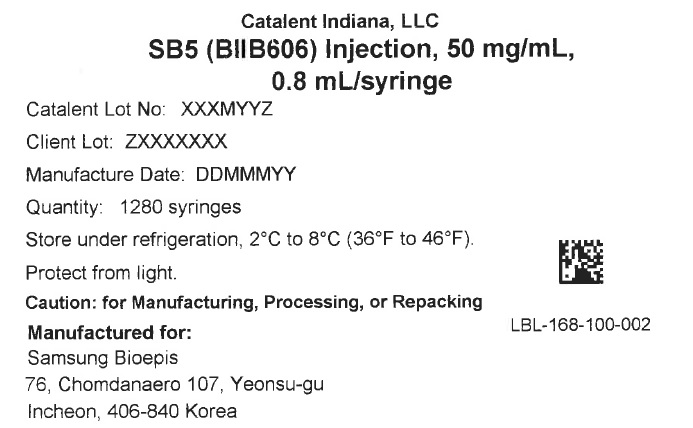
Label: SB5 (BIIB606)- adalimumab-bwwd injection, solution
- NDC Code(s): 61434-005-00
- Packager: Catalent Indiana, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SB5 (BIIB606)
adalimumab-bwwd injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61434-005 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADALIMUMAB-BWWD (UNII: FYS6T7F842) (ADALIMUMAB - UNII:FYS6T7F842) ADALIMUMAB-BWWD 40 mg in 0.8 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61434-005-00 1280 in 1 CASE 08/27/2017 1 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 08/27/2017 Labeler - Catalent Indiana, LLC (172209277)