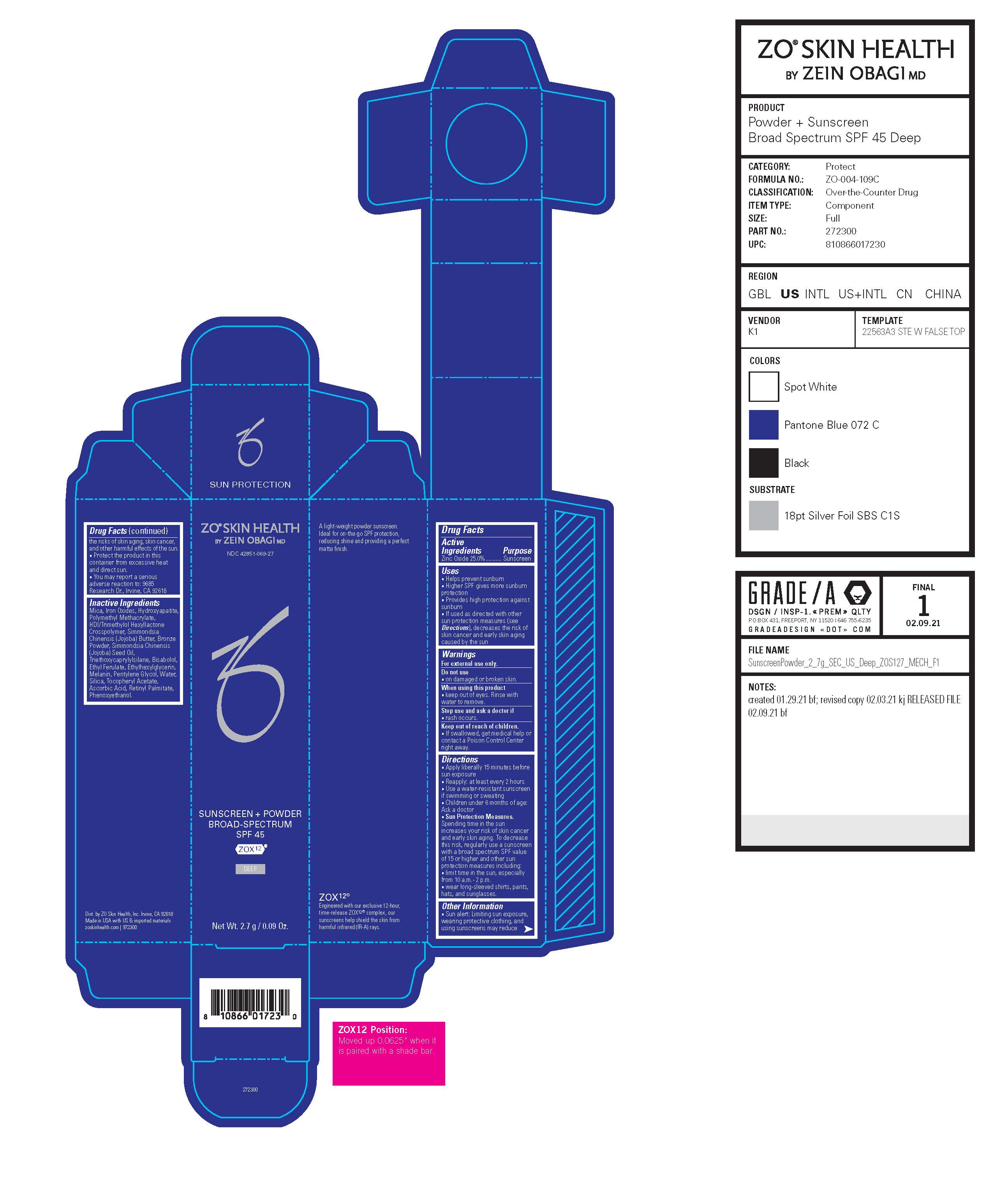
Label: SUNSCREEN PLUS POWDER BROAD-SPECTRUM SPF 45- zinc oxide powder
- NDC Code(s): 42851-069-27
- Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides high protection against sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply: at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: ▪ limit time in the sun, especially from 10 a.m.- 2 p.m. ▪ wear long-sleeved shirts, pants, hats, and sunglasses.
Other Information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
Inactive Ingredients
HDI/Trimethylol Hexyllactone Crosspolymer, Simmondsia Chinensis (Jojoba) Butter, Bronze Powder, Simmondsia Chinensis
(Jojoba) Seed Oil, Triethoxycaprylylsilane, Bisabolol, Ethyl Ferulate, Ethylhexylglycerin, Melanin, Pentylene Glycol, Water, Silica, Tocopheryl Acetate, Ascorbic Acid, Retinyl Palmitate, Phenoxyethanol. - SPL UNCLASSIFIED SECTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SUNSCREEN PLUS POWDER BROAD-SPECTRUM SPF 45
zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-069 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.675 mg in 2.7 g Inactive Ingredients Ingredient Name Strength HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) JOJOBA BUTTER (UNII: XIA46H803R) COPPER (UNII: 789U1901C5) JOJOBA OIL (UNII: 724GKU717M) ETHYL FERULATE (UNII: 5B8915UELW) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBIC ACID (UNII: PQ6CK8PD0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) LEVOMENOL (UNII: 24WE03BX2T) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-069-27 1 in 1 CARTON 02/09/2021 1 2.7 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/09/2021 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Bentley Laboratories, LLC 068351753 manufacture(42851-069)