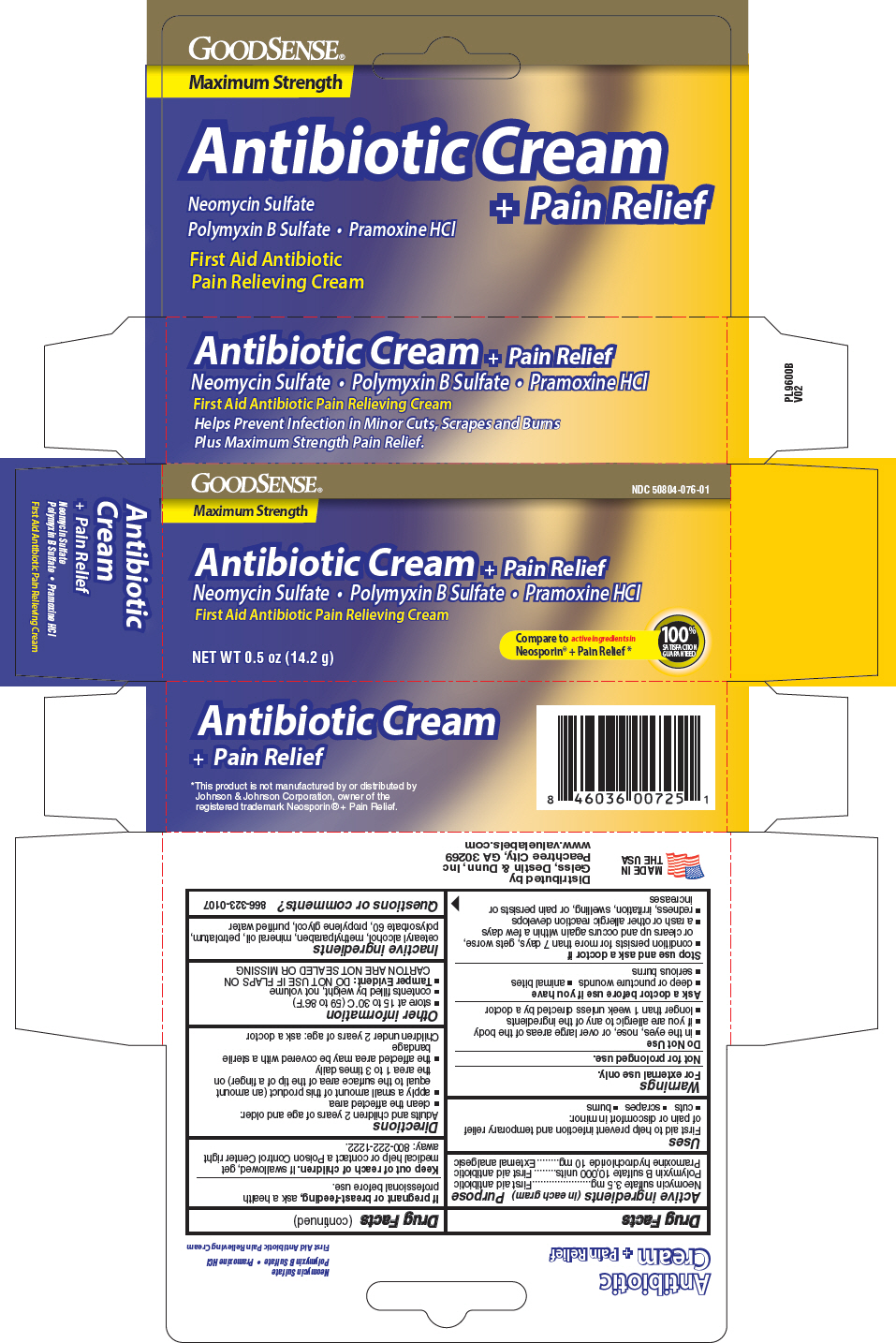
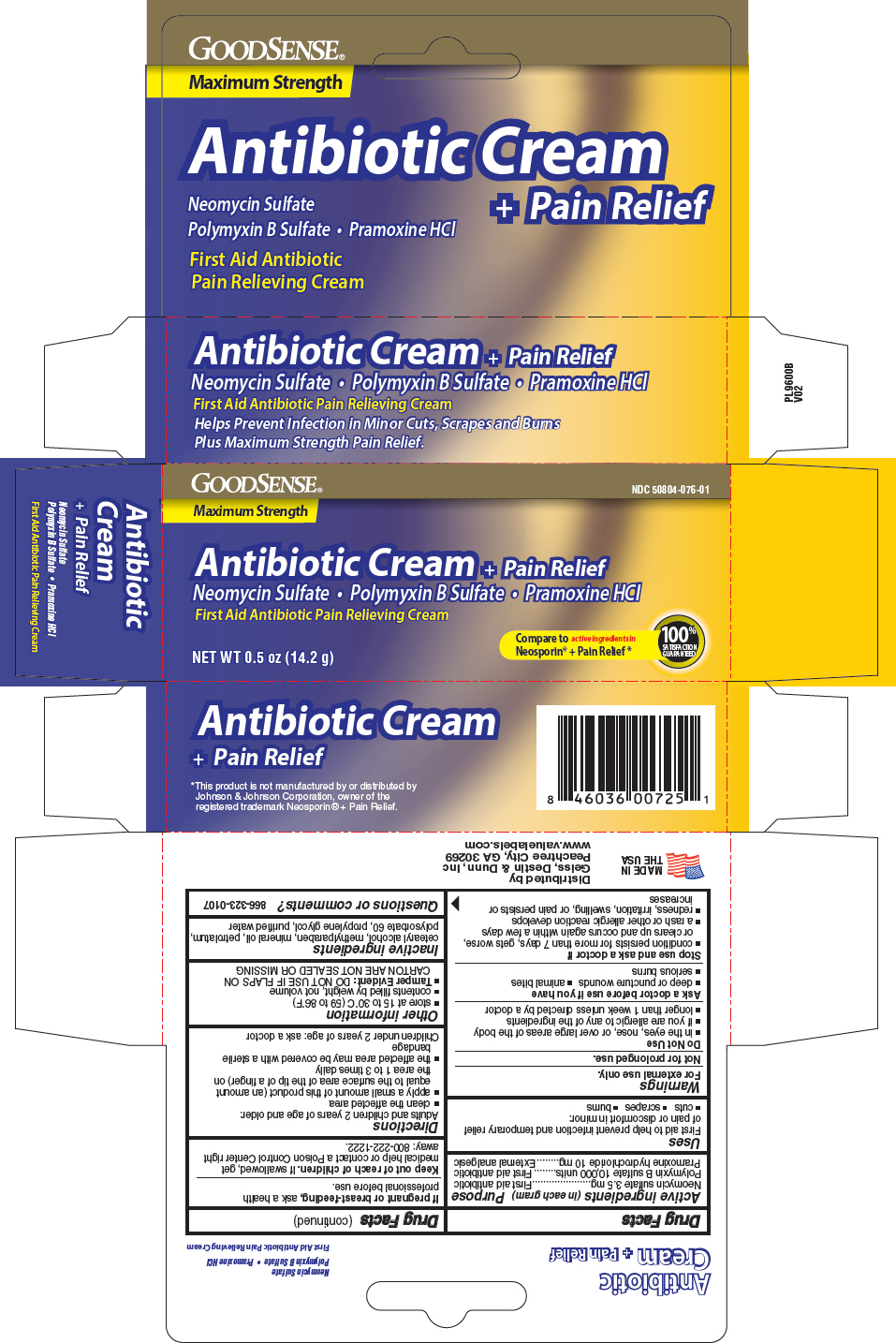
Label: GOODSENSE ANTIBIOTIC PLUS PAIN RELIEF- neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride cream
- NDC Code(s): 50804-076-01
- Packager: Geiss, Destin & Dunn, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only.
Not for prolonged use.
Do Not Use
- in the eyes, nose, or over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14.2 g Tube Carton
-
INGREDIENTS AND APPEARANCE
GOODSENSE ANTIBIOTIC PLUS PAIN RELIEF
neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50804-076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [iU] in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50804-076-01 1 in 1 CARTON 03/21/2012 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/21/2012 Labeler - Geiss, Destin & Dunn, Inc. (076059836) Registrant - Taro Pharmaceuticals U.S. A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Natureplex LLC 062808196 manufacture(50804-076)