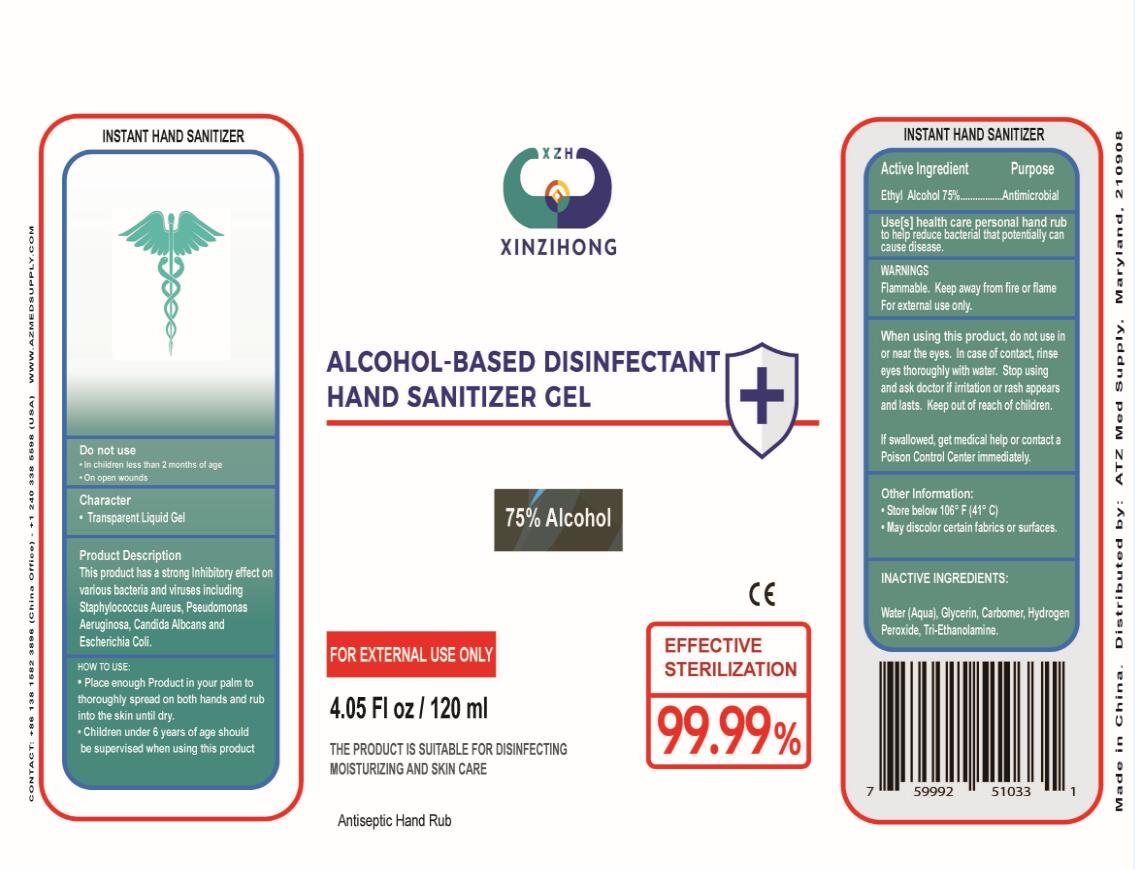
Label: ALCOHOL-BASED DISINFECTANT HAND SANITIZER gel
-
NDC Code(s):
74757-002-01,
74757-002-02,
74757-002-03,
74757-002-04, view more74757-002-05, 74757-002-06, 74757-002-07, 74757-002-08, 74757-002-09, 74757-002-10, 74757-002-11, 74757-002-12, 74757-002-13
- Packager: GUIZHOU XINZIHONG PHARMACEUTIC ADJUVANT CO.LTD
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 10, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- USE
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALCOHOL-BASED DISINFECTANT HAND SANITIZER
alcohol-based disinfectant hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74757-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) HYDROGEN PEROXIDE (UNII: BBX060AN9V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74757-002-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 2 NDC:74757-002-03 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 3 NDC:74757-002-02 330 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 4 NDC:74757-002-04 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 5 NDC:74757-002-05 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 6 NDC:74757-002-06 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 7 NDC:74757-002-07 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 8 NDC:74757-002-08 300 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 9 NDC:74757-002-09 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 10 NDC:74757-002-10 2000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 11 NDC:74757-002-11 3000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 12 NDC:74757-002-12 4000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 13 NDC:74757-002-13 5000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 03/20/2020 Labeler - GUIZHOU XINZIHONG PHARMACEUTIC ADJUVANT CO.LTD (540725476) Registrant - GUIZHOU XINZIHONG PHARMACEUTIC ADJUVANT CO.LTD (540725476) Establishment Name Address ID/FEI Business Operations GUIZHOU XINZIHONG PHARMACEUTIC ADJUVANT CO.LTD 540725476 manufacture(74757-002)