Label: IBUPROFEN tablet
- NDC Code(s): 36800-662-20
- Packager: TOPCO ASSOCIATES LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
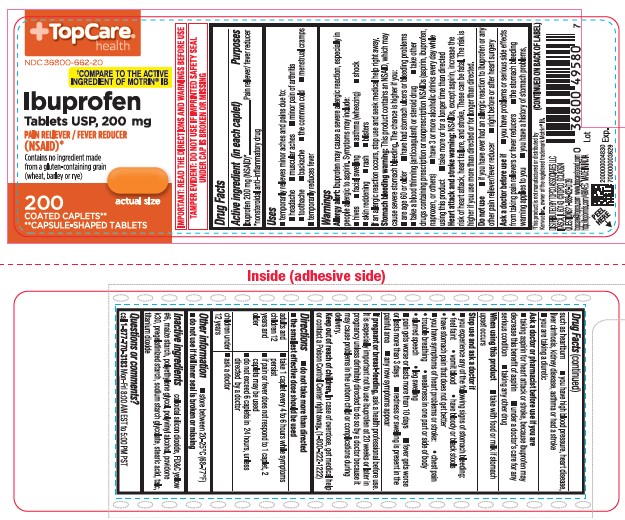
- Active ingredient (in each caplet)
- Purposes
- Uses
- Allergy alert:
-
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older have had stomach ulcers or bleeding problems
take a blood thinning (anticoagulant) or steroid drug take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
have 3 or more alcoholic drinks every day while using this product
take more or for a longer time than directed
- Heart attack and stroke warning:
- Do not use
-
Ask a doctor before use if
you have problems or serious side effects from taking pain relievers or fever reducers
the stomach bleeding warning applies to you
you have a history of stomach problems, such as heartburn
you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma or had a stroke
you are taking a diuretic - Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
• feel faint• vomit blood
• have bloody or black stools
• have stomach pain that does not get better
you have symptoms of heart problems or stroke:• chest pain
• trouble breathing• weakness in one part or side of body
• slurred speech• leg swelling
pain gets worse or lasts more than 10 days fever gets worse or lasts more than 3 days
redness or swelling is present in the painful area
any new symptoms appear
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
do not take more than directed
the smallest effective dose should be usedadults and children 12 years and older
take 1 caplet every 4 to 6 hours while symptoms persist
if pain or fever does not respond to 1 caplet, 2 caplets may be used
do not exceed 6 caplets in 24 hours, unless directed by a doctor
children under 12 years
ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-662 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE K30 (UNII: U725QWY32X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color orange Score no score Shape OVAL (Capsule shaped tablet) Size 14mm Flavor Imprint Code G;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-662-20 200 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202312 10/14/2024 Labeler - TOPCO ASSOCIATES LLC (006935977)