PROMACTA
-
eltrombopag olamine tablet
GlaxoSmithKline
----------
PROMACTA® (eltrombopag) Tablets
|
||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: RISK FOR HEPATOTOXICITY
PROMACTA may cause hepatotoxicity:
●Measure serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin prior to initiation of PROMACTA, every 2 weeks during the dose adjustment phase and monthly following establishment of a stable dose. If bilirubin is elevated, perform fractionation.
●Evaluate abnormal serum liver tests with repeat testing within 3 to 5 days. If the abnormalities are confirmed, monitor serum liver tests weekly until the abnormality(ies) resolve, stabilize, or return to baseline levels.
●Discontinue PROMACTA if ALT levels increase to ≥3X the upper limit of normal (ULN) and are:
- ●progressive, or
- ●persistent for ≥4 weeks, or
- ●accompanied by increased direct bilirubin, or
- ●accompanied by clinical symptoms of liver injury or evidence for hepatic decompensation.
Because of the risk for hepatotoxicity and other risks [see Warnings and Precautions (5.1-5.6)], PROMACTA is available only through a restricted distribution program called PROMACTA CARES. Under PROMACTA CARES, only prescribers, pharmacies, and patients registered with the program are able to prescribe, dispense, and receive PROMACTA. To enroll in PROMACTA CARES, call 1-877-9-PROMACTA [see Warnings and Precautions (5.8)].
1 INDICATIONS AND USAGE
PROMACTA is indicated for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. PROMACTA should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding. PROMACTA should not be used in an attempt to normalize platelet counts.
2 DOSAGE AND ADMINISTRATION
Only prescribers enrolled in PROMACTA CARES may prescribe PROMACTA [see Warnings and Precautions (5.8)].
Monitor liver tests (ALT, AST, and bilirubin) and complete blood counts (CBCs), including platelet counts and peripheral blood smears, prior to initiation of PROMACTA and throughout therapy with PROMACTA. If bilirubin is elevated, perform fractionation. Monitor CBCs, including platelet counts, for at least 4 weeks following discontinuation of PROMACTA [see Warnings and Precautions (5.3)]. In clinical studies, platelet counts generally increased within 1 to 2 weeks after starting PROMACTA and decreased within 1 to 2 weeks after discontinuing PROMACTA [see Clinical Studies (14)].
Use the lowest dose of PROMACTA to achieve and maintain a platelet count ≥50 x 109/L as necessary to reduce the risk for bleeding. Dose adjustments are based upon the platelet count response. Do not use PROMACTA in an attempt to normalize platelet counts [see Warnings and Precautions (5.4)].
Take PROMACTA on an empty stomach (1 hour before or 2 hours after a meal) [see Clinical Pharmacology (12.3)]. Allow at least a 4-hour interval between PROMACTA and other medications (e.g., antacids), calcium-rich foods (e.g., dairy products and calcium fortified juices), or supplements containing polyvalent cations such as iron, calcium, aluminum, magnesium, selenium, and zinc [see Drug Interactions (7.4) and Clinical Pharmacology (12.3)].
2.1 Initial Dose Regimen
Initiate PROMACTA at a dose of 50 mg once daily except in patients who are of East Asian ancestry or who have moderate to severe hepatic impairment.
For patients of East Asian ancestry (such as Chinese, Japanese, Taiwanese, or Korean), initiate PROMACTA at a reduced dose of 25 mg once daily [see Clinical Pharmacology (12.3)].
For patients with moderate or severe hepatic impairment, initiate PROMACTA at a reduced dose of 25 mg once daily [see Use in Specific Populations (8.6)].
2.2 Monitoring and Dose Adjustment
After initiating PROMACTA, adjust the dose to achieve and maintain a platelet count ≥50 x 109/L as necessary to reduce the risk for bleeding. Do not exceed a dose of 75 mg daily. Monitor clinical hematology and liver tests regularly throughout therapy with PROMACTA and modify the dosage regimen of PROMACTA based on platelet counts as outlined in Table 1. During therapy with PROMACTA, assess CBCs, including platelet count and peripheral blood smears, weekly until a stable platelet count has been achieved. Obtain CBCs including platelet counts and peripheral blood smears, monthly thereafter.
| Platelet Count Result | Dose Adjustment or Response |
| <50 x 109/L following at least 2 weeks of PROMACTA | Increase daily dose by 25 mg to a maximum of 75 mg/day. |
| ≥200 x 109/L to ≤400 x 109/L at any time | Decrease the daily dose by 25 mg. Wait 2 weeks to assess the effects of this and any subsequent dose adjustments. |
| >400 x 109/L |
Stop PROMACTA; increase the frequency of platelet monitoring to twice weekly. Once the platelet count is <150 x 109/L, reinitiate therapy at a daily dose reduced by 25 mg. |
| >400 x 109/L after 2 weeks of therapy at lowest dose of PROMACTA | Permanently discontinue PROMACTA. |
Modify the dosage regimen of concomitant ITP medications, as medically appropriate, to avoid excessive increases in platelet counts during therapy with PROMACTA. Do not administer more than one dose of PROMACTA within any 24-hour period.
2.3 Discontinuation
Discontinue PROMACTA if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks of therapy with PROMACTA at the maximum daily dose of 75 mg. Excessive platelet count responses, as outlined in Table 1, or important liver test abnormalities also necessitate discontinuation of PROMACTA [see Warnings and Precautions (5.1)].
3 DOSAGE FORMS AND STRENGTHS
25 mg tablets — round, biconvex, orange, film-coated tablets debossed with GS NX3 and 25 on one side. Each tablet, for oral administration, contains eltrombopag olamine, equivalent to 25 mg of eltrombopag free acid.
50 mg tablets — round, biconvex, blue, film-coated tablets debossed with GS UFU and 50 on one side. Each tablet, for oral administration, contains eltrombopag olamine, equivalent to 50 mg of eltrombopag free acid.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Hepatotoxicity
PROMACTA administration may cause hepatotoxicity. In the controlled clinical studies, one patient experienced Grade 4 (NCI Common Terminology Criteria for Adverse Events [NCI CTCAE] toxicity scale) elevations in serum liver test values during therapy with PROMACTA, worsening of underlying cardiopulmonary disease, and death. No patients in the placebo group experienced Grade 4 liver test abnormalities. Overall, serum liver test abnormalities (predominantly Grade 2 or less in severity) were reported in 10% and 8% of the PROMACTA and placebo groups, respectively. In the controlled studies, two patients (1%) treated with PROMACTA and two patients in the placebo group (3%) discontinued treatment due to hepatobiliary laboratory abnormalities. Seven of the patients treated with PROMACTA in the controlled studies with hepatobiliary laboratory abnormalities were re-exposed to PROMACTA in the extension study. Six of these patients again experienced liver test abnormalities (predominantly Grade 1) resulting in discontinuation of PROMACTA in one patient. In the extension study, one additional patient had PROMACTA discontinued due to liver test abnormalities (≤Grade 3).
Measure serum ALT, AST, and bilirubin prior to initiation of PROMACTA, every 2 weeks during the dose adjustment phase and monthly following establishment of a stable dose. If bilirubin is elevated, perform fractionation. Evaluate abnormal serum liver tests with repeat testing within 3 to 5 days. If the abnormalities are confirmed, monitor serum liver tests weekly until the abnormality(ies) resolve, stabilize, or return to baseline levels. Discontinue PROMACTA if ALT levels increase to ≥3X the upper limit of normal (ULN) and are:
- progressive, or
- persistent for ≥4 weeks, or
- accompanied by increased direct bilirubin, or
- accompanied by clinical symptoms of liver injury or evidence for hepatic decompensation.
Reinitiating treatment with PROMACTA is not recommended. If the potential benefit for reinitiating PROMACTA treatment is considered to outweigh the risk for hepatotoxicity, then cautiously reintroduce PROMACTA and measure serum liver tests weekly during the dose adjustment phase. If liver tests abnormalities persist, worsen or recur, then permanently discontinue PROMACTA.
Exercise caution when administering PROMACTA to patients with hepatic disease. Use a lower starting dose of PROMACTA in patients with moderate to severe hepatic disease and monitor closely [see Dosage and Administration (2.1)].
5.2 Bone Marrow Reticulin Formation and Risk for Bone Marrow Fibrosis
PROMACTA is a thrombopoietin (TPO) receptor agonist and TPO-receptor agonists increase the risk for development or progression of reticulin fiber deposition within the bone marrow.
In the extension study, seven patients had reticulin fiber deposition reported in bone marrow biopsies, including two patients who also had collagen fiber deposition. The fiber deposition was not associated with cytopenias and did not necessitate discontinuation of PROMACTA. However, clinical studies have not excluded a risk of bone marrow fibrosis with cytopenias.
Prior to initiation of PROMACTA, examine the peripheral blood smear closely to establish a baseline level of cellular morphologic abnormalities. Following identification of a stable dose of PROMACTA, examine peripheral blood smears and CBCs monthly for new or worsening morphological abnormalities (e.g., teardrop and nucleated red blood cells, immature white blood cells) or cytopenia(s). If the patient develops new or worsening morphological abnormalities or cytopenia(s), discontinue treatment with PROMACTA and consider a bone marrow biopsy, including staining for fibrosis.
5.3 Worsened Thrombocytopenia and Hemorrhage Risk After Cessation of PROMACTA
Discontinuation of PROMACTA may result in thrombocytopenia of greater severity than was present prior to therapy with PROMACTA. This worsened thrombocytopenia may increase the patient's risk of bleeding, particularly if PROMACTA is discontinued while the patient is on anticoagulants or antiplatelet agents. In the controlled clinical studies, transient decreases in platelet counts to levels lower than baseline were observed following discontinuation of treatment in 10% and 6% of the PROMACTA and placebo groups, respectively. Serious hemorrhagic events requiring the use of supportive ITP medications occurred in 3 severely thrombocytopenic patients within one month following the discontinuation of PROMACTA; none were reported among the placebo group.
Following discontinuation of PROMACTA, obtain weekly CBCs, including platelet counts for at least 4 weeks and consider alternative treatments for worsening thrombocytopenia, according to current treatment guidelines [see Adverse Reactions (6.1)].
5.4 Thrombotic/Thromboembolic Complications
Thrombotic/thromboembolic complications may result from excessive increases in platelet counts. Excessive doses of PROMACTA or medication errors that result in excessive doses of PROMACTA may increase platelet counts to a level that produces thrombotic/thromboembolic complications. In the controlled clinical studies, one thrombotic/thromboembolic complication was reported within the groups that received PROMACTA and none within the placebo groups. Seven patients experienced thrombotic/thromboembolic complications in the extension study. Use caution when administering PROMACTA to patients with known risk factors for thromboembolism (e.g., Factor V Leiden, ATIII deficiency, antiphospholipid syndrome, etc). To minimize the risk for thrombotic/thromboembolic complications, do not use PROMACTA in an attempt to normalize platelet counts. Follow the dose adjustment guidelines to achieve and maintain a platelet count of ≥50 x 109/L [see Dosage and Administration (2.2)].
5.5 Malignancies and Progression of Malignancies
PROMACTA stimulation of the TPO receptor on the surface of hematopoietic cells may increase the risk for hematologic malignancies. In the controlled clinical studies, patients were treated with PROMACTA for a maximum of 6 weeks and during this period no hematologic malignancies were reported. One hematologic malignancy (non-Hodgkin's lymphoma) was reported in the extension study. PROMACTA is not indicated for the treatment of thrombocytopenia due to causes of thrombocytopenia (e.g., myelodysplasia or chemotherapy) other than chronic ITP.
5.6 Laboratory Monitoring
Complete Blood Counts (CBCs): Monitor CBCs, including platelet counts and peripheral blood smears, prior to initiation, throughout, and following discontinuation of therapy with PROMACTA. Prior to the initiation of PROMACTA, examine the peripheral blood differential to establish the extent of red and white blood cell abnormalities. Obtain CBCs, including platelet counts and peripheral blood smears, weekly during the dose adjustment phase of therapy with PROMACTA and then monthly following establishment of a stable dose of PROMACTA. Obtain CBCs, including platelet counts, weekly for at least 4 weeks following discontinuation of PROMACTA [see Dosage and Administration (2) and Warnings and Precautions (5.2, 5.3)].
Liver tests: Monitor serum liver tests (ALT, AST, and bilirubin) prior to initiation of PROMACTA, every 2 weeks during the dose adjustment phase and monthly following establishment of a stable dose. If bilirubin is elevated, perform fractionation. If abnormal levels are detected, repeat the tests within 3 to 5 days. If the abnormalities are confirmed, monitor serum liver tests weekly until the abnormality(ies) resolve, stabilize, or return to baseline levels. Discontinue PROMACTA for the development of important liver test abnormalities [see Warnings and Precautions (5.1)].
5.7 Cataracts
In the controlled clinical studies, cataracts developed or worsened in five (5%) patients who received 50 mg PROMACTA daily and two (3%) placebo-group patients. In the extension study, cataracts developed or worsened in 4% of patients who underwent ocular examination prior to therapy with PROMACTA. Cataracts were observed in toxicology studies of eltrombopag in rodents [see Nonclinical Toxicology (13.2)]. Perform a baseline ocular examination prior to administration of PROMACTA and, during therapy with PROMACTA, regularly monitor patients for signs and symptoms of cataracts.
5.8 PROMACTA Distribution Program
PROMACTA is available only through a restricted distribution program called PROMACTA CARES. Under PROMACTA CARES, only prescribers, pharmacies, and patients registered with the program are able to prescribe, dispense, and receive PROMACTA. This program provides educational materials and a mechanism for the proper use of PROMACTA. To enroll in PROMACTA CARES, call 1-877-9-PROMACTA. Prescribers and patients are required to understand the risks of therapy with PROMACTA. Prescribers are required to understand the information in the prescribing information and be able to:
- Educate patients on the benefits and risks of treatment with PROMACTA, ensure that the patient receives the Medication Guide, instruct them to read it, and encourage them to ask questions when considering PROMACTA. Patients may be educated by the enrolled prescriber or a healthcare provider under that prescriber’s direction.
- Review the PROMACTA CARES Prescriber Enrollment Forms, sign the form, and return the form according to PROMACTA CARES Program instructions.
- As part of the initial prescription process for PROMACTA, obtain the patient's signature on the Patient Enrollment and Consent form, sign it, place the original signed form in the patient's medical record, send a copy to PROMACTA CARES, and give a copy to the patient.
- Report any serious adverse events associated with the use of PROMACTA to PROMACTA CARES Call Center at 1-877-9-PROMACTA or to the FDA’s MedWatch Program at 1-800-FDA-1088.
- Report serious adverse events observed in patients receiving PROMACTA, including events actively solicited at 6-month intervals.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
In clinical studies, hemorrhage was the most common serious adverse reaction and most hemorrhagic reactions followed discontinuation of PROMACTA. Other serious adverse reactions included liver test abnormalities and thrombotic/thromboembolic complications [see Warnings and Precautions (5.1, 5.2)].
The data described below reflect PROMACTA exposure to 313 patients with chronic ITP aged 18 to 85, of whom 65% were female. PROMACTA was studied in 2 randomized, placebo controlled studies in which patients received the drug for no more than 6 weeks. PROMACTA was also studied in an open label single arm study in which patients received the drug over an extended period of time. Overall, PROMACTA was administered to 81 patients for at least 6 months and 39 patients for at least 1 year.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 2 presents the most common adverse drug reactions (experienced by more than 1 patient receiving PROMACTA) from the placebo-controlled studies, with a higher incidence in PROMACTA versus placebo.
| Preferred Term |
PROMACTA 50mg n = 106 (%) |
Placebo n = 67 (%) |
| Nausea | 6 | 4 |
| Vomiting | 4 | 3 |
| Menorrhagia | 4 | 1 |
| Myalgia | 3 | 1 |
| Paresthesia | 3 | 1 |
| Cataract | 3 | 1 |
| Dyspepsia | 2 | 0 |
| Ecchymosis | 2 | 1 |
| Thrombocytopenia | 2 | 0 |
| Increased ALT | 2 | 0 |
| Increased AST | 2 | 0 |
| Conjunctival hemorrhage | 2 | 1 |
Among 207 patients with chronic ITP who received PROMACTA in the single-arm extended study, the adverse reactions occurred in a pattern similar to those reported in the placebo-controlled studies.
7 DRUG INTERACTIONS
7.1 Cytochrome P450
In vitro studies demonstrate that CYP1A2 and CYP2C8 are involved in the oxidative metabolism of eltrombopag. The significance of coadministration of PROMACTA with 1) moderate or strong inhibitors of CYP 1A2 (e.g., ciprofloxacin, fluvoxamine) and CYP 2C8 (e.g., gemfibrozil, trimethoprim); 2) inducers of CYP 1A2 (e.g., tobacco, omeprazole) and CYP 2C8 (e.g., rifampin); or 3) other substrates of these CYP enzymes on the systemic exposure of PROMACTA has not been established in clinical studies. Monitor patients for signs and symptoms of excessive eltrombopag exposure when PROMACTA is administered concomitantly with these moderate or strong inhibitors of CYP1A2 or CYP2C8.
7.2 Transporters
In vitro studies demonstrate that eltrombopag is an inhibitor of the organic anion transporting polypeptide OATP1B1 and can increase the systemic exposure of other drugs that are substrates of this transporter (e.g., benzylpenicillin, atorvastatin, fluvastatin, pravastatin, rosuvastatin, methotrexate, nateglinide, repaglinide, rifampin). In a clinical study of healthy adult subjects, administration of a single dose of rosuvastatin following repeated daily PROMACTA dosing increased plasma rosuvastatin AUC0-∞ by 55% and Cmax by 103% [see Clinical Pharmacology (12.3)].
Use caution when concomitantly administering PROMACTA and drugs that are substrates of OATP1B1. Monitor patients closely for signs and symptoms of excessive exposure to the drugs that are substrates of OATP1B1 and consider reduction of the dose of these drugs. In clinical trials with eltrombopag, a dose reduction of rosuvastatin by 50% was recommended for coadministration with eltrombopag.
7.3 UDP-glucuronosyltransferases (UGTs)
In vitro studies demonstrate that eltrombopag is an inhibitor of UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7, and UGT2B15, enzymes involved in the metabolism of multiple drugs, such as acetaminophen, narcotics, and nonsteroidal anti-inflammatory drugs (NSAIDs). The significance of this inhibition on the potential for increased systemic exposure of drugs that are substrates of these UGTs following coadministration with PROMACTA has not been evaluated in clinical studies. Monitor patients closely for signs or symptoms of excessive exposure to these drugs when concomitantly administered with PROMACTA.
In vitro studies demonstrate that UGT1A1 and UGT1A3 are responsible for the glucuronidation of PROMACTA. The significance of coadministration of PROMACTA with moderate or strong inhibitors or inducers on the systemic exposure of PROMACTA has not been evaluated in clinical studies. Monitor patients closely for signs or symptoms of excessive exposure to PROMACTA when concomitantly administered with these moderate or strong inhibitors of UGT1A1 or UGT1A3.
7.4 Polyvalent Cations (Chelation)
Eltrombopag chelates polyvalent cations (such as iron, calcium, aluminum, magnesium, selenium, and zinc) in foods, mineral supplements, and antacids. In a clinical study, administration of PROMACTA with a polyvalent cation-containing antacid (1,524 mg aluminum hydroxide, 1,425 mg magnesium carbonate, and sodium alginate) decreased plasma eltrombopag systemic exposure by approximately 70% [see Clinical Pharmacology (12.3)]. PROMACTA must not be taken within 4 hours of any medications or products containing polyvalent cations such as antacids, dairy products, and mineral supplements to avoid significant reduction in PROMACTA absorption due to chelation [see Dosage and Administration (2)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of eltrombopag use in pregnancy. In animal reproduction and developmental toxicity studies, there was evidence of embryolethality and reduced fetal weights at maternally toxic doses. PROMACTA should be used in pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Pregnancy Registry: A pregnancy registry has been established to collect information about the effects of PROMACTA during pregnancy. Physicians are encouraged to register pregnant patients, or pregnant women may enroll themselves in the PROMACTA pregnancy registry by calling 1-888-825-5249.
In an early embryonic development study, female rats received eltrombopag at doses of 0.8, 2, and 7 times the human clinical exposure (based on AUC). Increased pre- and post-implantation loss and reduced fetal weight were observed at the highest dose which also caused maternal toxicity.
In an embryofetal development study, pregnant rats received eltrombopag at doses of 0.8, 2, and 7 times the human clinical exposure (based on AUC). Decreased fetal weights and a slight increase in the presence of cervical ribs were observed at the highest dose which also caused maternal toxicity. However, no evidence of major structural malformations was observed.
In an embryofetal development study in pregnant rabbits treated with oral eltrombopag doses of 0.1, 0.3, and 0.6 times the human clinical exposure (based on AUC) no evidence of fetotoxicity, embryolethality, or teratogenicity was observed.
In a pre- and post-natal developmental toxicity study in pregnant rats (F0), no adverse effects on maternal reproductive function or on the development of the offspring (F1) were observed at doses up to 2 times the human clinical exposure (based on AUC). Eltrombopag was detected in the plasma of offspring (F1). The plasma concentrations in pups increased with dose (0.8 and 2 times the human clinical exposure based on AUC) following administration of drug to the F0 dams.
8.3 Nursing Mothers
It is not known whether eltrombopag is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from PROMACTA, a decision should be made whether to discontinue nursing or to discontinue PROMACTA taking into account the importance of PROMACTA to the mother and the known benefits of nursing.
8.4 Pediatric Use
The safety and efficacy of PROMACTA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 106 patients in 2 randomized clinical studies of PROMACTA 50 mg dose, 22% were 65 years of age and older, and 9% were 75 years of age and older. No overall differences in safety or efficacy have been observed between older and younger patients in the placebo-controlled studies, but greater sensitivity of some older individuals cannot be ruled out. In general, dose adjustment for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
The disposition of PROMACTA was compared in patients with hepatic impairment to subjects with normal hepatic function. Apparent clearance of PROMACTA was reduced by approximately 50% in patients with moderate and severe (as indicated by the Child-Pugh method) hepatic impairment. In this clinical study that did not evaluate protein binding effects, the half-life of PROMACTA was prolonged 2-fold in patients with moderate and severe hepatic impairment.
For patients with moderate and severe hepatic impairment, initiate PROMACTA at a reduced dose of 25 mg once daily [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
8.7 Renal Impairment
The safety and efficacy of PROMACTA in patients with varying degrees of renal function have not been established. Closely monitor patients with impaired renal function when administering PROMACTA.
10 OVERDOSAGE
In the event of overdose, platelet counts may increase excessively and result in thrombotic/thromboembolic complications. In case of an overdose, consider oral administration of a metal cation-containing preparation, such as calcium, aluminum, or magnesium preparations to chelate eltrombopag and thus limit absorption. Closely monitor platelet counts. Reinitiate treatment with PROMACTA in accordance with dosing and administration recommendations [see Dosage and Administration (2.2)].
In one report, a subject ingested 5,000 mg of PROMACTA and was treated with gastric lavage, oral lactulose, intravenous fluids, omeprazole, atropine, furosemide, calcium, dexamethasone, and plasmapheresis. The patient’s platelet count increased to a maximum of 929 x 109/L at 13 days following the ingestion. The patient also experienced rash, bradycardia, ALT/AST elevations, and fatigue. The abnormal platelet count and liver test abnormalities persisted for 3 weeks. After 2 months follow-up, all events had resolved without sequelae.
Hemodialysis is not expected to enhance the elimination of PROMACTA because eltrombopag is not significantly renally excreted and is highly bound to plasma proteins.
11 DESCRIPTION
PROMACTA (eltrombopag) Tablets contain eltrombopag olamine, a small molecule thrombopoietin (TPO) receptor agonist for oral administration. Eltrombopag interacts with the transmembrane domain of the TPO receptor (also known as cMpl) leading to increased platelet production. Each tablet contains eltrombopag olamine in the amount equivalent to 25 mg or 50 mg of eltrombopag free acid.
Eltrombopag olamine is a biphenyl hydrazone. The chemical name for eltrombopag olamine is 3'-{(2Z)-2-[1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene]hydrazino}-2'-hydroxy-3-biphenylcarboxylic acid - 2-aminoethanol (1:2). It has the molecular formula C25H22N4O4.2(C2H7NO). The molecular weight is 564.65 for eltrombopag olamine and 442.5 for eltrombopag free acid. Eltrombopag olamine has the following structural formula:
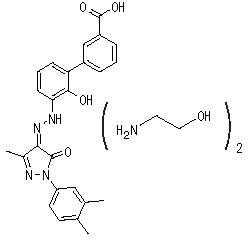
Eltrombopag olamine is practically insoluble in aqueous buffer across a pH range of 1 to 7.4, and is sparingly soluble in water.
The inactive ingredients of PROMACTA are: Tablet Core: magnesium stearate, mannitol, microcrystalline cellulose, povidone, and sodium starch glycolate. Coating: hypromellose, polyethylene glycol 400, titanium dioxide, and FD&C Yellow No. 6 aluminum lake (25 mg tablet) or FD&C Blue No. 2 aluminum lake (50 mg tablet).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism Of Action
Eltrombopag is an orally bioavailable, small-molecule TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor and initiates signaling cascades that induce proliferation and differentiation of megakaryocytes from bone marrow progenitor cells.
12.2 Pharmacodynamics
ECG Effects: There is no indication of a QT/QTc prolonging effect of PROMACTA in doses up to 150 mg daily for 5 days. The effects of PROMACTA at doses up to 150 mg daily for 5 days (supratherapeutic doses) on the QT/QTc interval was evaluated in a double-blind, randomized, placebo- and positive-controlled (moxifloxacin 400 mg, single oral dose) crossover trial in healthy adult subjects. Assay sensitivity was confirmed by significant QTc prolongation by moxifloxacin.
12.3 Pharmacokinetics
A population pharmacokinetic model analysis suggests that the pharmacokinetic profile for eltrombopag following oral administration is best described by a 2-compartment model. Based on this model, the estimated exposures of eltrombopag after administration to patients with ITP are shown in Table 3.
| Regimen of PROMACTA |
AUC(0-τ) (mcg.hr/mL) |
| 50 mg once daily (N = 34) |
91.9 (73.6, 115) |
| 75 mg once daily (N = 26) |
146 (122, 176) |
Absorption: Eltrombopag is absorbed with a peak concentration occurring 2 to 6 hours after oral administration. Based on urinary excretion and biotransformation products eliminated in feces, the oral absorption of drug-related material following administration of a single 75 mg solution dose was estimated to be at least 52%.
In a clinical study, administration of a single 75 mg-dose of PROMACTA with a polyvalent cation-containing antacid (1,524 mg aluminum hydroxide, 1,425 mg magnesium carbonate, and sodium alginate) decreased plasma eltrombopag AUC0-∞ and Cmax by 70%. The contribution of sodium alginate to this interaction is not known [see Drug Interactions (7.4)].
An open-label, randomized, crossover study was conducted to assess the effect of food on the bioavailability of eltrombopag. A standard high-fat breakfast significantly decreased plasma eltrombopag AUC0-∞ by approximately 59% and Cmax by 65% and delayed tmax by 1 hour. The calcium content of this meal may have also contributed to this decrease in exposure.
Distribution: The concentration of eltrombopag in blood cells is approximately 50-79% of plasma concentrations based on a radiolabel study. In vitro studies suggest that eltrombopag is highly bound to human plasma proteins (>99%). Eltrombopag is not a substrate for p-glycoprotein (Pgp) or OATP1B1.
Metabolism: Absorbed eltrombopag is extensively metabolized, predominantly through pathways including cleavage, oxidation, and conjugation with glucuronic acid, glutathione, or cysteine. In a human radiolabel study, eltrombopag accounted for approximately 64% of plasma radiocarbon AUC0-∞. Metabolites due to glucuronidation and oxidation were also detected. In vitro studies suggest that CYP 1A2 and 2C8 are responsible for the oxidative metabolism of eltrombopag. UGT1A1 and UGT1A3 are responsible for the glucuronidation of eltrombopag.
Elimination: The predominant route of eltrombopag excretion is via feces (59%), and 31% of the dose is found in the urine. Unchanged eltrombopag in feces accounts for approximately 20% of the dose; unchanged eltrombopag is not detectable in urine. The plasma elimination half-life of eltrombopag is approximately 21 to 32 hours in healthy subjects and 26-35 hours in ITP patients.
Race: Based on both non-compartment analysis and population pharmacokinetic analysis, plasma eltrombopag exposure was approximately 70% higher in some Asian subjects of Japanese, Chinese, Taiwanese, and Korean ancestry (i.e., East Asian) with ITP as compared to non-Asian subjects who were predominantly Caucasian [see Dosage and Administration (2.1)]. In addition, the pharmacodynamic (PD) response to eltrombopag was qualitatively similar in the Asian subjects, but the absolute PD response was somewhat greater.
An approximately 40% higher systemic eltrombopag exposure in healthy African-American subjects was noted in at least one clinical pharmacology study. The effect of African-American ethnicity on exposure and related safety and efficacy of eltrombopag has not been established.
Gender: Results from a population pharmacokinetic model suggest that males have a 27% greater apparent eltrombopag clearance than females, after adjustment for the body weight difference.
Hepatic Impairment: Plasma eltrombopag pharmacokinetics in subjects with mild, moderate, and severe hepatic impairment compared to healthy subjects was investigated following administration of a single 50 mg dose of eltrombopag. The degree of hepatic impairment was based on Child-Pugh score. Plasma eltrombopag AUC0-∞ was 41% higher in subjects with mild hepatic impairment, and 80% to 93% higher in subjects with moderate to severe hepatic impairment compared with healthy subjects. A corresponding reduction in apparent clearance was also reported. The impact of hepatic impairment was highly variable between subjects. Unbound eltrombopag (active) concentrations for this highly protein bound drug was not measured [see Dosage and Administration (2.1) and Use in Specific Populations (8.6)].
Renal Impairment: The pharmacokinetics of eltrombopag have not been established in patients with renal impairment [see Use in Specific Populations (8.7)].
Drug Interactions:Cytochrome P450: In vitro studies report that eltrombopag is an inhibitor of CYP2C8 and CYP2C9 as measured using paclitaxel and diclofenac as the probe substrates. A clinical study where PROMACTA 75 mg once daily was administered for 7 days to 24 healthy male subjects did not show inhibition or induction of the metabolism of a combination of probe substrates for CYP 1A2 (caffeine), CYP2C19 (omeprazole), CYP2C9 (flurbiprofen), or CYP3A4 (midazolam) in humans. Probe substrates for CYP2C8 were not evaluated in this study.
In vitro studies suggest that CYP 1A2 and 2C8 are responsible for oxidative metabolism of eltrombopag. Clinical studies evaluating the effect of strong inducers or inhibitors of these CYP enzymes responsible for the metabolism of eltrombopag have not been conducted.
Transporters: In vitro studies demonstrated that eltrombopag is an inhibitor of the OATP1B1. Administration of 75 mg of PROMACTA once daily for 5 days with a single 10 mg-dose of the OATP1B1 substrate, rosuvastatin, to 39 healthy adult subjects increased plasma rosuvastatin AUC0-∞ by 55% and Cmax by 103% [see Drug Interactions (7.2)].
UDP-glucuronosyltransferases (UGTs): See Drug Interactions (7.3).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Eltrombopag does not stimulate platelet production in rats, mice, or dogs because of unique TPO receptor specificity. Data from these animals do not fully model effects in humans.
Eltrombopag was not carcinogenic in mice at doses up to 75 mg/kg/day or in rats at doses up to 40 mg/kg/day (exposures up to 4 and 5 times the human clinical exposure based on AUC, respectively).
Eltrombopag was not mutagenic or clastogenic in a bacterial mutation assay or in 2 in vivo assays in rats (micronucleus and unscheduled DNA synthesis, 11 times the human clinical exposure based on Cmax). In the in vitro mouse lymphoma assay, eltrombopag was marginally positive (<3-fold increase in mutation frequency).
Eltrombopag did not affect female fertility in rats at doses up to 20 mg/kg/day (2 times the human clinical exposure based on AUC). Eltrombopag did not affect male fertility in rats at doses up to 40 mg/kg/day, the highest dose tested (5 times the human clinical exposure based on AUC).
13.2 Animal Pharmacology/Toxicology
Eltrombopag is phototoxic and photoclastogenic in vitro. In vitro photoclastogenic effects were observed only at cytotoxic drug concentrations (≥15 mcg/mL) and at UV light exposure intensity (30 MED, minimal erythematous dose). No evidence of in vitro photoclastogenicity was observed at higher drug concentrations (up to 58.4 mcg/mL) and UV light exposure of 15 MED. There was no evidence of in vivo cutaneous phototoxicity in mice, photo-ocular toxicity in rats or photo-ocular toxicity in mice at exposures up to 11, 6, and 7 times the human clinical exposure based on AUC, respectively.
Treatment-related cataracts were detected in rodents in a dose- and time-dependent manner. At ≥7 times the human clinical exposure based on AUC, cataracts were observed in mice after 6 weeks and in rats after 28 weeks of dosing. At ≥5 times the human clinical exposure based on AUC, cataracts were observed in mice after 13 weeks and in rats after 39 weeks of dosing. Cataracts were not observed in dogs after 52 weeks of dosing (3 times the human clinical exposure based on AUC). The clinical relevance of these findings is unknown [see Warnings and Precautions (5.7)].
Renal tubular toxicity was observed in studies up to 14 days in duration in mice and rats at exposures that were generally associated with morbidity and mortality. Tubular toxicity was also observed in a 2-year oral carcinogenicity study in mice at doses of 25, 75, and 150 mg/kg/day. The exposure at the lowest dose was 1.4 times the human clinical exposure based on AUC. No similar effects were observed after 13 weeks at exposures greater than those associated with renal changes in the 2-year study, suggesting that this effect is both dose- and time-dependent. Renal tubular toxicity was not observed in rats in a 2-year carcinogenicity study or in dogs after 52 weeks at exposures 5 and 3 times the human clinical exposure based on AUC, respectively.
Eltrombopag produced hepatocellular hypertrophy in mice (7 times the human clinical exposure based on AUC), rats (5 times the human clinical exposure based on AUC), rabbits (1.4 times the human clinical exposure based on AUC), and dogs (4 times the human clinical exposure based on AUC) and hepatocellular vacuolation in rats (2 times the human clinical exposure based on AUC).
13.3 Reproductive and Developmental Toxicology
Eltrombopag was administered orally to pregnant rats in an embryofetal development study at 10, 20, or 60 mg/kg/day (0.8, 2, and 7 times the human clinical exposure, respectively, based on AUC). Decreases in maternal body weight gain and food consumption occurred in the 60 mg/kg/day dose group. At this maternally toxic dose, male and female fetal weights were significantly reduced (6% to 7%) and there was a slight increase in the presence of cervical ribs, a fetal variation.
In an embryofetal development study in mated female rabbits, eltrombopag was administered orally at 30, 80, or 150 mg/kg/day (0.1, 0.3, and 0.6 times the human clinical exposure, respectively, based on AUC). There was no evidence of fetotoxicity, embryolethality, or teratogenicity at any dose.
In a pre- and post-natal developmental toxicity study in pregnant rats (F0), no adverse effects on maternal reproductive function or on the development of the offspring (F1) were observed at doses up to 2 times the human clinical exposure (based on AUC). Eltrombopag was detected in the plasma of offspring (F1). The plasma concentrations in pups increased with dose (0.8 and 2 times the human clinical exposure based on AUC) following administration of drug to the F0 dams.
14 CLINICAL STUDIES
The efficacy and safety of PROMACTA in adult patients with chronic ITP were evaluated in 2 randomized double-blind, placebo-controlled studies and in an open-label extension study.
14.1 Studies 1 and 2
In studies 1 and 2, patients who had completed at least one prior ITP therapy and who had a platelet count <30 x 109/L were randomized to either daily placebo or PROMACTA administered over a maximum treatment period of 6 weeks, followed by 6 weeks off therapy. During the studies, PROMACTA or placebo were discontinued if the platelet count exceeded 200 x 109/L. The primary efficacy endpoint was response rate, defined as a shift from a baseline platelet count of <30 x 109/L to ≥50 x 109/L at any time during the treatment period.
The median age of the patients was 50 years and 60% were female. Approximately 70% of the patients had received at least 2 prior ITP therapies (predominantly corticosteroids, immunoglobulins, rituximab, cytotoxic therapies, danazol, and azathioprine) and 40% of the patients had undergone splenectomy. The median baseline platelet counts (approximately 18 x 109/L) were similar among all treatment groups.
Study 1 randomized 114 patients (2:1) to PROMACTA 50 mg or placebo. Study 2 randomized 117 patients (1:1:1:1) among placebo or one of three dose regimens of PROMACTA, 30 mg, 50 mg, or 75 mg each administered daily.
Table 4 shows the outcomes for the placebo groups and the groups of patients who received the 50 mg daily regimen of PROMACTA.
| Study |
PROMACTA 50 mg Daily | Placebo |
| 1 | 43/73 (59%)* | 6/37 (16%) |
| 2 | 19/27 (70%)* | 3/27 (11%) |
*p <0.001 for PROMACTA versus placebo.
The platelet count response to PROMACTA was similar among patients who had or had not undergone splenectomy. In general, increases in platelet counts were detected 1 week following initiation of PROMACTA and the maximum response observed after 2 weeks of therapy. Within the placebo and 50 mg dose group of PROMACTA, the study drug was discontinued due to an increase in platelet counts to >200 x 109/L in 3% and 27% of the patients, respectively. The median duration of treatment with the 50 mg dose of PROMACTA in Study 1 was 42 days and Study 2 was 43 days.
Of seven patients (three in the placebo group and four in the group that received PROMACTA) who underwent hemostatic challenges, additional ITP medications were required in all placebo group patients and none of the patients treated with PROMACTA. Surgical procedures accounted for most of the hemostatic challenges. Hemorrhage requiring transfusion occurred in one placebo group patient and no patients treated with PROMACTA.
14.2 Extension Study
Patients who completed any prior clinical study with PROMACTA were enrolled in an open label, single arm study in which attempts were made to decrease the dose or eliminate the need for any concomitant ITP medications. PROMACTA was administered to 109 patients; 74 completed 3 months of treatment, 53 completed 6 months and three patients completed 1 year of therapy. The median baseline platelet count was 18 x 109/L prior to administration of PROMACTA. Median platelet counts at 3, 6, and 9 months on study were 74 x 109/L, 67 x 109/L, and 95 x 109/L, respectively. The median daily dose of PROMACTA following 6 months of therapy was 50 mg (n = 53); the median daily dose was also 50 mg among patients with no change in the dose regimen of PROMACTA over 2 months or more of therapy (n = 45).
16 HOW SUPPLIED/STORAGE AND HANDLING
The 25 mg tablets are round, biconvex, orange, film-coated tablets debossed with GS NX3 and 25 on one side and are available in bottles of 30: NDC 0007-4640-13.
The 50 mg tablets are round, biconvex, blue, film-coated tablets debossed with GS UFU and 50 on one side and are available in bottles of 30: NDC 0007-4641-13.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Medication Guide.
17.1 Information for Patients
Prior to treatment, patients should fully understand the risks and benefits of PROMACTA. Inform patients that the risks associated with long-term administration of PROMACTA are unknown and that they must enroll in PROMACTA CARES, which provides for the proper use of PROMACTA in ITP patients.
Inform patients of the following risks and considerations for PROMACTA:
- Therapy with PROMACTA is administered to achieve and maintain a platelet count ≥50 x 109/L as necessary to reduce the risk for bleeding; PROMACTA is not used to normalize platelet counts.
- Therapy with PROMACTA may be associated with hepatobiliary laboratory abnormalities. Monitor serum liver tests (ALT, AST, and bilirubin) prior to initiation of PROMACTA, every 2 weeks during the dose adjustment phase and monthly following establishment of a stable dose. If bilirubin is elevated, perform fractionation.
- Inform patients that they should report any of the following signs and symptoms of liver problems to their healthcare provider right away.
- yellowing of the skin or the whites of the eyes (jaundice),
- unusual darkening of the urine,
- unusual tiredness,
- right upper stomach area pain.
- Following discontinuation of PROMACTA, thrombocytopenia and risk of bleeding may develop that is worse than that experienced prior to therapy with PROMACTA, particularly if PROMACTA is discontinued while the patient is on anticoagulants or antiplatelet agents.
- Therapy with PROMACTA increases the risk of reticulin fiber formation within the bone marrow, and further fiber formation may progress to marrow fibrosis. Detection of peripheral blood cell abnormalities may necessitate a bone marrow examination.
- Too much PROMACTA may result in excessive platelet counts and a risk for thrombotic/thromboembolic complications.
- PROMACTA stimulates certain bone marrow cells to make platelets and may increase the risk for progression of underlying MDS or hematological malignancies.
- Platelet counts and CBCs, including peripheral blood smears, must be performed weekly until a stable dose of PROMACTA has been achieved; thereafter, platelet counts and CBCs, including peripheral blood smears, must be performed monthly while taking PROMACTA.
- Patients must be closely monitored with weekly platelet counts and CBCs for at least 4 weeks following discontinuation of PROMACTA.
- Even during therapy with PROMACTA, patients should continue to avoid situations or medications that may increase the risk for bleeding.
- Patients must be advised to keep at least a 4 hour interval between PROMACTA and foods, mineral supplements, and antacids which contain polyvalent cations such as iron, calcium, aluminum, magnesium, selenium, and zinc.
PROMACTA is a registered trademark of GlaxoSmithKline.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2008, GlaxoSmithKline. All rights reserved.
MEDICATION GUIDE
PROMACTA®(pro-MAC-ta)
(eltrombopag)
Tablets
Read the Medication Guide that comes with PROMACTA before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about PROMACTA?
PROMACTA can cause uncommon but serious side effects:
-
Liver problems. PROMACTA may damage your liver and cause serious illness and death. You must have blood tests to check your liver before you start taking PROMACTA and during treatment with PROMACTA. Your healthcare provider will order these blood tests. In some cases PROMACTA treatment may need to be stopped. Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems:
- yellowing of the skin or the whites of the eyes (jaundice),
- unusual darkening of the urine,
- unusual tiredness,
- right upper stomach area pain.
- Bone marrow changes (increased reticulin and possible bone marrow fibrosis). Long-term use of PROMACTA may cause changes in your bone marrow. These changes may lead to abnormal blood cells or your body making less blood cells. The mild form of these bone marrow changes is called “increased reticulin”. It is not known if this may progress to a more severe form called “fibrosis.” The mild form may cause no problems while the severe form may cause life-threatening blood problems. Signs of bone marrow changes may show up as abnormal results in your blood tests. Your healthcare provider will decide if abnormal blood test results mean that you should have bone marrow tests or if you should stop taking PROMACTA.
- Worsening low blood platelet count (thrombocytopenia) and risk of bleeding shortly after stopping PROMACTA. When you stop taking PROMACTA, your low blood platelet count (thrombocytopenia) may become worse than before you started taking PROMACTA. These effects are most likely to happen within 4 weeks after you stop taking PROMACTA. The lower platelet counts during this time period may increase your risk of bleeding, especially if you take a blood thinner or other medicines that affects platelets. Your healthcare provider will check your blood platelet counts for at least 4 weeks after you stop taking PROMACTA. Call your healthcare provider right away to report any bruising or bleeding.
- High platelet counts and higher chance for blood clots. You have a higher chance of getting a blood clot if your platelet count is too high during treatment with PROMACTA. You may have severe complications or die from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your healthcare provider will check your blood platelet counts, and change your dose or stop PROMACTA if your platelet counts get too high.
- Worsening of blood cancers. PROMACTA is not for use in patients with blood cancer or a precancerous condition called myelodysplastic syndrome (MDS). If you have one of these conditions, PROMACTA may worsen your cancer or condition and may cause you to die sooner.
When you are being treated with PROMACTA, your healthcare provider will closely monitor your dose of PROMACTA and blood tests, including platelet counts and liver tests.
PROMACTA is available only through a program called “PROMACTA CARES”. To receive PROMACTA, you must talk to your healthcare provider, understand the benefits and risks of PROMACTA and agree to enroll into PROMACTA CARES.
- During therapy with PROMACTA, your healthcare provider may change your dose of PROMACTA, depending upon the change in your blood platelet count. You must have blood platelet count tests done before, during and after your therapy with PROMACTA.
- PROMACTA is used to try to keep your platelet count about 50,000 per microliter in order to lower your risk for bleeding. PROMACTA is not used to make your platelet count normal.
See “What are the possible side effects of PROMACTA?” for other side effects of PROMACTA.
What is PROMACTA?
PROMACTA is a prescription medicine used to treat low blood platelet counts in adults with chronic immune (idiopathic) thrombocytopenic purpura (ITP), when other medicines to treat your ITP or surgery to remove the spleen have not worked well enough.
PROMACTA is only:
- prescribed by healthcare providers who are enrolled in PROMACTA CARES.
- given to people who are enrolled in PROMACTA CARES.
It is not known if PROMACTA works or if it is safe in people under the age of 18 years.
PROMACTA is for treatment of certain people with low platelet counts caused by chronic ITP, not low platelet counts caused by other conditions or diseases.
What should I tell my healthcare provider before taking PROMACTA?
Tell your healthcare provider if you:
- have liver problems
- have or had a blood clot
- have a history of cataracts
- have had surgery to remove your spleen (splenectomy)
- have a bone marrow problem, including a blood cancer or Myelodysplastic Syndrome (MDS)
- have bleeding problems
- are Asian and you are of Chinese, Japanese, Taiwanese, or Korean ancestry, you may need a lower dose of PROMACTA.
- are pregnant, think you may be pregnant, or plan to get pregnant. It is not known if PROMACTA will harm an unborn baby.
Pregnancy Registry: There is a registry for women who become pregnant during treatment with PROMACTA. If you become pregnant, consider this registry. The purpose of the registry is to collect safety information about the health of you and your baby. Contact the registry as soon as you become aware of the pregnancy, or ask your healthcare provider to contact the registry for you. You and your healthcare provider can get information and enroll in the registry by calling 1-888-825-5249. - are breast-feeding or plan to breast-feed. It is not known if PROMACTA passes into your breast milk. You and your healthcare provider should decide whether you will take PROMACTA or breast-feed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. PROMACTA may affect the way certain medicines work. Certain other medicines may affect the way PROMACTA works.
Especially tell your healthcare provider if you take:
- certain medicines used to treat high cholesterol, called “statins”.
- a blood thinner medicine.
Certain medicines may keep PROMACTA from working correctly. Take PROMACTA either 4 hours before or 4 hours after taking these products:
- antacids used to treat stomach ulcers or heartburn.
- multivitamins or products that contain iron, calcium, aluminum, magnesium, selenium, and zinc which may be found in mineral supplements.
Ask your healthcare provider if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take PROMACTA?
To receive PROMACTA, you must first talk with your healthcare provider and understand the benefits and risks of PROMACTA. You must agree to and follow all of the instructions in PROMACTA CARES.
- Before you can begin to receive PROMACTA, your healthcare provider will:
- explain PROMACTA CARES to you.
- answer all of your questions about PROMACTA and PROMACTA CARES.
- make sure you read this PROMACTA Medication Guide.
- have you sign the PROMACTA CARES Patient Enrollment Form.
- Take PROMACTA exactly as your healthcare provider tells you. Do not stop using PROMACTA without talking with your healthcare provider first. Do not change your dose or schedule for taking PROMACTA unless your healthcare provider tells you to change it.
- Take PROMACTA on an empty stomach, either 1 hour before or 2 hours after eating food.
- Take PROMACTA at least 4 hours before or 4 hours after eating dairy products and calcium fortified juices.
- If you miss a dose of PROMACTA, wait and take your next scheduled dose. Do not take more than one dose of PROMACTA in one day.
- If you take too much PROMACTA, you may have a higher chance of serious side effects. Call your healthcare provider right away.
- Your healthcare provider will check your platelet count every week and change your dose of PROMACTA as needed. This will happen every week until your healthcare provider decides that your dose of PROMACTA can stay the same. After that, you will need to have blood tests every month. When you stop taking PROMACTA, you will need to have blood tests for at least 4 weeks to check if your platelet count drops too low.
- Tell your healthcare provider about any bruising or bleeding that happens while you take and after you stop taking PROMACTA.
What should I avoid while taking PROMACTA?
Avoid situations and medicines that may increase your risk of bleeding.
What are the possible side effects of PROMACTA?
Promacta may cause serious side effects.
- See “What is the most important information I should know about PROMACTA?”.
- New or worsened cataracts (a clouding of the lens in the eye). New or worsened cataracts have happened in people taking PROMACTA. Your healthcare provider will check your eyes before and during your treatment with PROMACTA. Tell your healthcare provider about any changes in your eyesight while taking PROMACTA.
The most common side effects of PROMACTA are:
- nausea
- vomiting
- heavy or longer than normal menstrual periods
- muscle aches
- abnormal skin sensations such as tingling, itching, or burning
- indigestion
- bruising
- bleeding into the tissue that covers the eye and under side of the eyelid (conjunctiva).
These are not all the possible side effects of PROMACTA. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PROMACTA Tablets?
- Store at room temperature, 59oF to 86oF (15oC to 30oC).
- Keep PROMACTA and all medicines out of the reach of children.
What are the ingredients in PROMACTA?
Active Ingredient: eltrombopag olamine.
Inactive Ingredients:
- Tablet Core: Magnesium stearate, mannitol, microcrystalline cellulose, povidone, and sodium starch glycolate.
- Coating: Hypromellose, polyethylene glycol 400, titanium dioxide, and FD&C Yellow No. 6 aluminum lake (25 mg tablet) or FD&C Blue No. 2 aluminum lake (50 mg tablet).
General information about the safe and effective use of PROMACTA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PROMACTA for a condition for which it was not prescribed. Do not give PROMACTA to other people even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about PROMACTA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about PROMACTA that is written for healthcare professionals. For more information you can call toll-free 1-888-825-5249.
PROMACTA is a registered trademark of GlaxoSmithKline.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2008, GlaxoSmithKline. All rights reserved.
Revised: October 2008
PRM:1MG
| PROMACTA
eltrombopag olamine tablet |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| PROMACTA
eltrombopag olamine tablet |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 12/2008GlaxoSmithKline