SPRYCEL
-
dasatinib tablet
Bristol-Myers Squibb
----------
|
|||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SPRYCEL® (dasatinib) is indicated for the treatment of adults with chronic, accelerated, or myeloid or lymphoid blast phase chronic myeloid leukemia (CML) with resistance or intolerance to prior therapy including imatinib. The effectiveness of SPRYCEL is based on hematologic and cytogenetic response rates [see Clinical Studies (14)]. There are no controlled trials demonstrating a clinical benefit, such as improvement in disease-related symptoms or increased survival.
SPRYCEL is also indicated for the treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
The recommended starting dosage of SPRYCEL (dasatinib) for chronic phase CML is 100 mg administered orally once daily (QD), either in the morning or in the evening. The recommended starting dosage of SPRYCEL for accelerated phase CML, myeloid or lymphoid blast phase CML, or Ph+ ALL is 140 mg/day administered orally in two divided doses (70 mg twice daily [BID]), one in the morning and one in the evening. Tablets should not be crushed or cut; they should be swallowed whole. SPRYCEL can be taken with or without a meal.
In clinical studies, treatment with SPRYCEL was continued until disease progression or until no longer tolerated by the patient. The effect of stopping treatment after the achievement of a complete cytogenetic response (CCyR) has not been investigated.
2.1 Dose Modification
Concomitant Strong CYP3A4 inducers: The use of concomitant strong CYP3A4 inducers may decrease dasatinib plasma concentrations and should be avoided (eg, dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, phenobarbital). St. John's Wort may decrease dasatinib plasma concentrations unpredictably and should be avoided. If patients must be coadministered a strong CYP3A4 inducer, based on pharmacokinetic studies, a SPRYCEL dose increase should be considered. If the dose of SPRYCEL is increased, the patient should be monitored carefully for toxicity [see Drug Interactions (7.2)].
Concomitant Strong CYP3A4 inhibitors: CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole) may increase dasatinib plasma concentrations. Grapefruit juice may also increase plasma concentrations of dasatinib and should be avoided.
Selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is recommended. If SPRYCEL must be administered with a strong CYP3A4 inhibitor, a dose decrease to 20 mg daily should be considered. If 20 mg/day is not tolerated, either the strong CYP3A4 inhibitor must be discontinued, or SPRYCEL should be stopped until treatment with the inhibitor has ceased. When the strong inhibitor is discontinued, a washout period of approximately 1 week should be allowed before the SPRYCEL dose is increased. [See Drug Interactions (7.1).]
2.2 Dose Escalation
In clinical studies of adult CML and Ph+ ALL patients, dose escalation to 140 mg once daily (chronic phase CML) or 100 mg twice daily (advanced phase CML and Ph+ ALL) was allowed in patients who did not achieve a hematologic or cytogenetic response at the recommended starting dosage.
2.3 Dose Adjustment for Adverse Reactions
Myelosuppression
In clinical studies, myelosuppression was managed by dose interruption, dose reduction, or discontinuation of study therapy. Hematopoietic growth factor has been used in patients with resistant myelosuppression. Guidelines for dose modifications are summarized in Table 1.
| * ANC: absolute neutrophil count | ||
| Chronic
Phase CML (starting dose 100 mg once daily) | ANC* <0.5× 109/L or Platelets <50× 109/L | 1. Stop SPRYCEL until ANC ≥1.0 × 109/L and platelets ≥50 × 109/L. |
| 2. Resume treatment with SPRYCEL at the original starting dose if recovery occurs in ≤7 days. | ||
| 3. If platelets<25 × 109/L or recurrence of ANC <0.5 × 109/L for >7 days, repeat Step 1 and resume SPRYCEL at a reduced dose of 80 mg once daily (second episode) or discontinue (third episode). | ||
| Accelerated
Phase CML, Blast Phase CML and Ph+ ALL (starting dose 70 mg twice daily) | ANC<0.5 × 109/L or Platelets<10 × 109/L | 1. Check if cytopenia is related to leukemia (marrow aspirate or biopsy). |
| 2. If cytopenia is unrelated to leukemia, stop SPRYCEL until ANC ≥1.0 × 109/L and platelets≥20 × 109/L and resume at the original starting dose. | ||
| 3. If recurrence of cytopenia, repeat Step 1 and resume SPRYCEL at a reduced dose of 50 mg twice daily (second episode) or 40 mg twice daily (third episode). | ||
| 4. If cytopenia is related to leukemia, consider dose escalation to 100 mg twice daily. | ||
Non-hematological adverse reactions
If a severe non-hematological adverse reaction develops with SPRYCEL use, treatment must be withheld until the event has resolved or improved. Thereafter, treatment can be resumed as appropriate at a reduced dose depending on the initial severity of the event.
3 DOSAGE FORMS AND STRENGTHS
SPRYCEL (dasatinib) Tablets are available as 20-mg, 50-mg, 70-mg, and 100-mg white to off-white, biconvex, film-coated tablets. [See How Supplied (16.1).]
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Treatment with SPRYCEL is associated with severe (NCI CTC Grade 3 or 4) thrombocytopenia, neutropenia, and anemia. Their occurrence is more frequent in patients with advanced phase CML or Ph+ ALL than in chronic phase CML. Complete blood counts should be performed weekly for the first 2 months and then monthly thereafter, or as clinically indicated. Myelosuppression was generally reversible and usually managed by withholdingSPRYCEL temporarily or dose reduction [see Dosage and Administration (2.3) and Adverse Reactions (6.1)]. In a Phase 3 dose-optimization study in patients with chronic phase CML, Grade 3 or 4 myelosuppression was reported less frequently in patients treated with 100 mg once daily than in patients treated with 70 mg twice daily [see Table 6, Adverse Reactions (6.1)].
5.2 Bleeding Related Events
In addition to causing thrombocytopenia in human subjects, dasatinib caused platelet dysfunction in vitro. In all clinical studies, severe central nervous system (CNS) hemorrhages, including fatalities, occurred in <1% of patients receiving SPRYCEL. Severe gastrointestinal hemorrhage occurred in 4% of patients and generally required treatment interruptions and transfusions. Other cases of severe hemorrhage occurred in 2% of patients. Most bleeding events were associated with severe thrombocytopenia [see Adverse Reactions (6.1)].
Patients were excluded from participation in SPRYCEL clinical studies if they took medications that inhibit platelet function or anticoagulants. In some trials, the use of anticoagulants, aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs) was allowed concurrently with SPRYCEL if the platelet count was >50,000. Caution should be exercised if patients are required to take medications that inhibit platelet function or anticoagulants.
5.3 Fluid Retention
SPRYCEL is associated with fluid retention. In all clinical studies, severe fluid retention was reported in 8% of patients, including pleural and pericardial effusion reported in 5% and 1% of patients, respectively. Severe ascites and generalized edema were each reported in <1% of patients. Severe pulmonary edema was reported in 1% of patients [see Adverse Reactions (6.1)]. Patients who develop symptoms suggestive of pleural effusion such as dyspnea or dry cough should be evaluated by chest X-ray. Severe pleural effusion may require thoracentesis and oxygen therapy. Fluid retention events were typically managed by supportive care measures that include diuretics or short courses of steroids.
5.4 QT Prolongation
In vitro data suggest that dasatinib has the potential to prolong cardiac ventricular repolarization (QT interval). In single-arm clinical studies in patients with leukemia treated with SPRYCEL, the mean QTc interval changes from baseline using Fridericia’s method (QTcF) were 3–6 msec; the upper 95% confidence intervals for all mean changes from baseline were <8 msec. Nine patients had QTc prolongation reported as an adverse event. Three patients (<1%) experienced a QTcF >500 msec.
SPRYCEL should be administered with caution to patients who have or may develop prolongation of QTc. These include patients with hypokalemia or hypomagnesemia, patients with congenital long QT syndrome, patients taking anti-arrhythmic medicines or other medicinal products that lead to QT prolongation, and cumulative high-dose anthracycline therapy. Hypokalemia or hypomagnesemia should be corrected prior to SPRYCEL administration.
5.5 Pregnancy
Pregnancy Category D: SPRYCEL may cause fetal harm when administered to a pregnant woman. In nonclinical studies, at plasma concentrations below those observed in humans receiving therapeutic doses of dasatinib, fetal toxicity was observed in rats and rabbits. Fetal death was observed in rats. In both rats and rabbits, the lowest doses of dasatinib tested (rat: 2.5 mg/kg/day [15 mg/m2/day] and rabbit: 0.5 mg/kg/day [6 mg/m2/day]) resulted in embryo-fetal toxicities. These doses produced maternal AUCs of 105 ng•hr/mL (0.3-fold the human AUC in females at a dose of 70 mg twice daily) and 44 ng•hr/mL (0.1-fold the human AUC) in rats and rabbits, respectively. Embryo-fetal toxicities included skeletal malformations at multiple sites (scapula, humerus, femur, radius, ribs, clavicle), reduced ossification (sternum; thoracic, lumbar, and sacral vertebrae; forepaw phalanges; pelvis; and hyoid body), edema, and microhepatia.
Women should be advised of the potential hazard to the fetus and to avoid becoming pregnant. If SPRYCEL is used during pregnancy, or if the patient becomes pregnant while taking SPRYCEL, the patient should be apprised of the potential hazard to the fetus.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling.
- Myelosuppression [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Bleeding related events [see Warnings and Precautions (5.2)].
- Fluid retention [see Warnings and Precautions (5.3)].
- QT prolongation [see Warnings and Precautions (5.4)].
6.1 Clinical Studies Experience
The data described below reflect exposure to SPRYCEL in 2182 patients with leukemia in clinical studies (starting dosage 100 mg once daily, 140 mg once daily, 50 mg twice daily, or 70 mg twice daily). The median duration of therapy was 11 months (range 0.03–26 months).
The majority of SPRYCEL-treated patients experienced adverse reactions at some time. Drug was discontinued for adverse reactions in 9% of patients in chronic phase CML, 10% in accelerated phase CML, 15% in myeloid blast phase CML, and 8% in lymphoid blast phase CML or Ph+ ALL. In a Phase 3 dose-optimization study in patients with chronic phase CML, the rate of discontinuation for adverse reaction was lower in patients treated with 100 mg once daily than in patients treated with 70 mg twice daily (4% and 12%, respectively).
The most frequently reported adverse reactions (reported in ≥20% of patients) included fluid retention events, diarrhea, headache, skin rash, nausea, hemorrhage, fatigue, and dyspnea.
The most frequently reported serious adverse reactions included pleural effusion (9%), pyrexia (3%), pneumonia (3%), infection (2%), febrile neutropenia (4%), gastrointestinal bleeding (4%), dyspnea (3%), sepsis (1%), diarrhea (2%), congestive heart failure (2%), and pericardial effusion (1%).
All adverse reactions (excluding laboratory abnormalities) that were reported in at least 10% of the patients in SPRYCEL clinical studies are shown in Table 2.
| Preferred Term | All
Patients (n=2182) | Chronic Phasea (n=1150) | Accelerated Phase (n=502) | Myeloid Blast Phase (n=280) | Lymphoid Blast Phase and Ph+ ALL (n=250) |
|
|---|---|---|---|---|---|---|
| All Grades | Grades 3/4 | Grades 3/4 | Grades 3/4 | Grades 3/4 | Grades 3/4 | |
| Percent (%) of Patients | ||||||
| a The chronic phase data include patients prescribed any dose of SPRYCEL. For selected adverse reactions in patients with chronic phase CML receiving the recommended 100 mg once daily starting dose, see Table 4. | ||||||
| b Includes left ventricular dysfunction, cardiac failure, cardiac failure congestive, cardiomyopathy, congestive cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and ventricular failure. | ||||||
| c Includes erythema, erythema multiforme, exfoliative rash, generalized erythema, heat rash, milia, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, skin exfoliation, skin irritation, systemic lupus erythematosus rash, urticaria vesiculosa, and rash vesicular. | ||||||
| Fluid Retention | 37 | 8 | 6 | 7 | 13 | 7 |
| Superficial localized edema | 20 | <1 | <1 | 1 | 1 | <1 |
| Pleural effusion | 22 | 5 | 4 | 5 | 10 | 6 |
| Other fluid retention | 10 | 3 | 3 | 3 | 6 | 2 |
| Generalized edema | 3 | <1 | <1 | 1 | <1 | 1 |
| Congestive heart failure/cardiac dysfunctionb | 2 | 1 | 2 | <1 | 2 | 1 |
| Pericardial effusion | 3 | 1 | 1 | 1 | 2 | 0 |
| Pulmonary edema | 2 | 1 | 1 | 1 | 1 | 1 |
| Ascites | <1 | <1 | 0 | 0 | 1 | <1 |
| Pulmonary hypertension | 1 | <1 | <1 | 0 | 1 | 1 |
| Diarrhea | 31 | 3 | 3 | 4 | 5 | 4 |
| Headache | 24 | 1 | 1 | 1 | 1 | 2 |
| Skin Rashc | 22 | 1 | 1 | 1 | 1 | 1 |
| Nausea | 22 | 1 | 1 | 1 | 2 | 2 |
| Hemorrhage | 21 | 6 | 2 | 11 | 12 | 8 |
| Gastrointestinal bleeding | 7 | 4 | 1 | 8 | 9 | 5 |
| CNS bleeding | 1 | <1 | 0 | <1 | <1 | 2 |
| Fatigue | 21 | 2 | 2 | 3 | 1 | 2 |
| Dyspnea | 20 | 4 | 5 | 4 | 5 | 2 |
| Musculoskeletal Pain | 14 | 1 | 2 | 1 | 1 | <1 |
| Pyrexia | 13 | 1 | 1 | 2 | 3 | 1 |
| Vomiting | 13 | 1 | 1 | 1 | 1 | 2 |
| Abdominal Pain | 10 | 1 | 1 | <1 | 1 | 2 |
In a Phase 2 randomized study of chronic phase CML, 101 patients received SPRYCEL (starting dosage 70 mg twice daily) and 49 patients received imatinib (starting dosage 800 mg daily [400 mg twice daily]). Crossover to the alternate therapy was permitted in this study. The median duration of therapy prior to crossover was longer for SPRYCEL (19 months) than for imatinib (3 months). Selected adverse reactions are presented in Table 3.
| Preferred Term | SPRYCELa
(n=101) | Imatiniba
(n=49) |
||
|---|---|---|---|---|
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Percent (%) of Patients | ||||
| a Starting dosage: SPRYCEL 70 mg twice daily; imatinib 800 mg daily (400 mg twice daily). | ||||
| b Includes left ventricular dysfunction, cardiac failure, cardiac failure congestive, cardiomyopathy, congestive cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and ventricular failure. | ||||
| Diarrhea | 37 | 2 | 29 | 2 |
| Fluid Retention | 36 | 7 | 43 | 0 |
| Pleural effusion | 23 | 5 | 0 | 0 |
| Superficial localized edema | 17 | 1 | 41 | 0 |
| Generalized edema | 2 | 0 | 4 | 0 |
| Congestive heart failure/ cardiac dysfunctionb | 2 | 1 | 0 | 0 |
| Pericardial effusion | 1 | 0 | 0 | 0 |
| Pulmonary edema | 3 | 2 | 0 | 0 |
| Pulmonary hypertension | 1 | 0 | 0 | 0 |
| Nausea | 24 | 0 | 33 | 0 |
| Hemorrhage | 18 | 1 | 8 | 0 |
| Gastrointestinal bleeding | 3 | 1 | 0 | 0 |
| Vomiting | 10 | 0 | 24 | 0 |
In the Phase 3 dose-optimization study in patients with chronic phase CML, the median duration of therapy was approximately 12 months (range <1–20 months). Selected adverse reactions are shown by dose regimen in Table 4.
| Preferred Term | 100
mg QD (n=165) | 140
mg QDa
(n=163) | 50
mg BIDa
(n=167) | 70
mg BIDa
(n=167) |
||||
|---|---|---|---|---|---|---|---|---|
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Percent (%) of Patients | ||||||||
| a Not
a recommended starting dosage of SPRYCEL for chronic phase CML. b Includes left ventricular dysfunction, cardiac failure, cardiac failure congestive, cardiomyopathy, congestive cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and ventricular failure. |
||||||||
| Diarrhea | 23 | 1 | 26 | 3 | 26 | 3 | 25 | 4 |
| Fluid Retention | 24 | 2 | 33 | 4 | 27 | 4 | 32 | 5 |
| Superficial localized edema | 14 | 0 | 14 | 1 | 14 | 0 | 16 | 0 |
| Pleural effusion | 10 | 2 | 20 | 2 | 16 | 3 | 18 | 2 |
| Generalized edema | 2 | 0 | 3 | 0 | 0 | 0 | 1 | 0 |
| Congestive heart failure/cardiac dysfunctionb | 0 | 0 | 2 | 1 | 1 | 1 | 4 | 2 |
| Pericardial effusion | 1 | 1 | 4 | 1 | 2 | 1 | 2 | 1 |
| Pulmonary edema | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 |
| Pulmonary hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hemorrhage | 10 | 1 | 12 | 1 | 9 | 2 | 14 | 2 |
| Gastrointestinal bleeding | 1 | 1 | 2 | 0 | 4 | 2 | 4 | 2 |
Laboratory Abnormalities
Myelosuppression was commonly reported in all patient populations. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anemia was higher in patients with advanced phase CML or Ph+ ALL than in chronic phase CML (Table 5). Myelosuppression was reported in patients with normal baseline laboratory values as well as in patients with pre-existing laboratory abnormalities.
In patients who experienced severe myelosuppression, recovery generally occurred following dose interruption or reduction; permanent discontinuation of treatment occurred in 5% of patients [see Warnings and Precautions (5.1)].
Grade 3 or 4 elevations of transaminase or bilirubin and Grade 3 or 4 hypocalcemia and hypophosphatemia were reported in patients with all phases of CML but were reported with an increased frequency in patients with myeloid or lymphoid blast phase CML and Ph+ ALL. Elevations in transaminase or bilirubin were usually managed with dose reduction or interruption. Patients developing Grade 3 or 4 hypocalcemia during the course of SPRYCEL therapy often had recovery with oral calcium supplementation.
In the Phase 2 randomized study, the frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anemia was 63%, 56%, and 19%, respectively, in the SPRYCEL (dasatinib) group and 39%, 14%, and 8%, respectively, in the imatinib group. The frequency of Grade 3 or 4 hypocalcemia was 4% in the SPRYCEL group and 0% in the imatinib group. Laboratory abnormalities reported in the Phase 3 dose-optimization study in patients with chronic phase CML are shown in Table 6.
| Chronic
Phasea
(n=1150) | Accelerated
Phase (n=502) | Myeloid
Blast Phase (n=280) | Lymphoid
Blast Phase and Ph+ ALL (n=250) |
|
|---|---|---|---|---|
| Percent (%) of Patients | ||||
| a The chronic phase data include patients prescribed any dose of SPRYCEL. For laboratory abnormalities in patients with chronic phase CML receiving the recommended 100 mg once daily starting dose, see Table 6. | ||||
| CTC grades: neutropenia (Grade 3≥0.5–1.0 × 109/L, Grade 4 <0.5 × 109/L); thrombocytopenia (Grade 3 ≥10–50 × 109/L, Grade 4 <10 × 109/L); anemia (hemoglobin ≥65–80 g/L, Grade 4 <65 g/L); elevated creatinine (Grade 3 >3–6 × upper limit of normal range (ULN), Grade 4 >6 × ULN); elevated bilirubin (Grade 3 >3–10 × ULN, Grade 4 >10 × ULN); elevated SGOT or SGPT (Grade 3 >5–20 × ULN, Grade 4 >20 × ULN); hypocalcemia (Grade 3 <7.0–6.0 mg/dL, Grade 4 <6.0 mg/dL); hypophosphatemia (Grade 3 <2.0–1.0 mg/dL, Grade 4 <1.0 mg/dL). | ||||
| Hematology Parameters | ||||
| Neutropenia | 46 | 68 | 80 | 78 |
| Thrombocytopenia | 41 | 71 | 81 | 78 |
| Anemia | 18 | 55 | 75 | 45 |
| Biochemistry Parameters | ||||
| Hypophosphatemia | 10 | 12 | 19 | 20 |
| Hypocalcemia | 2 | 7 | 16 | 11 |
| Elevated SGPT (ALT) | 1 | 3 | 6 | 7 |
| Elevated SGOT (AST) | 1 | 1 | 4 | 5 |
| Elevated Bilirubin | 1 | 1 | 4 | 5 |
| Elevated Creatinine | 1 | 2 | 3 | 1 |
| 100
mg QD (n=165) | 140 mg QDa
(n=163) | 50 mg BIDa
(n=167) | 70 mg BIDa
(n=167) |
|
|---|---|---|---|---|
| Percent (%) of Patients | ||||
| a Not a recommended starting dosage of SPRYCEL for chronic phase CML. | ||||
| CTC grades: neutropenia (Grade 3 ≥0.5–1.0 × 109/L, Grade 4 <0.5 × 109/L); thrombocytopenia (Grade 3 ≥10–50 × 109/L, Grade 4 <10 × 109/L); anemia (hemoglobin ≥65–80 g/L, Grade 4 <65 g/L); elevated creatinine (Grade 3 >3–6 × upper limit of normal range (ULN), Grade 4 >6 × ULN); elevated bilirubin (Grade 3 >3–10 × ULN, Grade 4 >10 × ULN); elevated SGOT or SGPT (Grade 3 >5–20 × ULN, Grade 4 >20 × ULN); hypocalcemia (Grade 3 <7.0–6.0 mg/dL, Grade 4 <6.0 mg/dL); hypophosphatemia (Grade 3 <2.0–1.0 mg/dL, Grade 4 <1.0 mg/dL). | ||||
| Hematology Parameters | ||||
| Neutropenia | 34 | 43 | 46 | 43 |
| Thrombocytopenia | 22 | 40 | 34 | 38 |
| Anemia | 10 | 19 | 18 | 17 |
| Biochemistry Parameters | ||||
| Hypophosphatemia | 8 | 6 | 7 | 7 |
| Hypocalcemia | 2 | 3 | 1 | 2 |
| Elevated SGPT (ALT) | 0 | 1 | 1 | 1 |
| Elevated SGOT (AST) | 1 | 1 | 0 | 0 |
| Elevated Bilirubin | 1 | 2 | 0 | 1 |
| Elevated Creatinine | 0 | 1 | 0 | 1 |
Additional Data From Clinical Trials
The following adverse reactions were reported in patients in the SPRYCEL clinical studies at a frequency of <10% (1%–<10%, 0.1%–<1%, or <0.1%). These events are included on the basis of clinical relevance.
Gastrointestinal Disorders: 1%–<10% – mucosal inflammation (including mucositis/stomatitis), dyspepsia, abdominal distension, constipation, gastritis, oral soft tissue disorder, colitis (including neutropenic colitis); 0.1%–<1% – dysphagia, anal fissure, upper gastrointestinal ulcer, pancreatitis; <0.1% – esophagitis.
General Disorders and Administration Site Conditions: 1%–<10% – asthenia, pain, chest pain, chills; 0.1%–<1% – malaise; <0.1% – temperature intolerance.
Skin and Subcutaneous Tissue Disorders: 1%–<10% – pruritus, acne, alopecia, dry skin, hyperhidrosis, urticaria, dermatitis (including eczema); 0.1%–<1% – skin ulcer, bullous conditions, pigmentation disorder, nail disorder, photosensitivity, acute febrile neutrophilic dermatosis, panniculitis; <0.1% – palmar-plantar erythrodysesthesia syndrome.
Respiratory, Thoracic, and Mediastinal Disorders: 1%–<10% – cough, lung infiltration, pneumonitis; 0.1%–<1% – asthma, bronchospasm; <0.1% – acute respiratory distress syndrome.
Nervous System Disorders:1%–<10% – neuropathy (including peripheral neuropathy), dizziness, dysgeusia, somnolence; 0.1%–<1% – tremor, syncope, amnesia; <0.1% – convulsion, cerebrovascular accident, transient ischemic attack.
Blood and Lymphatic System Disorders:1%–<10% – febrile neutropenia, pancytopenia; <0.1% – aplasia pure red cell.
Musculoskeletal and Connective Tissue Disorders:1%–<10% – arthralgia, myalgia, muscle inflammation, muscular weakness; 0.1%–<1% – musculoskeletal stiffness, rhabdomyolysis; <0.1% – tendonitis.
Investigations:1%–<10% – weight decreased, weight increased; 0.1%–<1% – blood creatine phosphokinase increased.
Infections and Infestations:1%–<10% – infections (including bacterial, viral, fungal, non-specified), pneumonia (including bacterial, viral, and fungal), upper respiratory tract infection/inflammation, herpes virus infection, enterocolitis infection; 0.1%–<1% – sepsis (including fatal outcomes).
Metabolism and Nutrition Disorders:1%–<10% – anorexia, appetite disturbances; 0.1%–<1% – hyperuricemia.
Cardiac Disorders:1%–<10% – arrhythmia (including tachycardia), palpitations; 0.1%–<1% – angina pectoris, cardiomegaly, pericarditis, ventricular arrhythmia (including ventricular tachycardia), myocardial infarction; <0.1% – myocarditis, acute coronary syndrome.
Eye Disorders:1%–<10% – visual disorder, dry eye; 0.1% –<1% – conjunctivitis.
Vascular Disorders:1%–<10% – flushing, hypertension; 0.1%–<1% – hypotension, thrombophlebitis; <0.1% – livedo reticularis.
Psychiatric Disorders:1%–<10% – insomnia, depression; 0.1%–<1% – anxiety, affect lability, confusional state, libido decreased.
Reproductive System and Breast Disorders:0.1%–<1% – gynecomastia, menstruation irregular.
Injury, Poisoning, and Procedural Complications:1%–<10% – contusion.
Ear and Labyrinth Disorders: 0.1%–<1% – tinnitus, vertigo.
Hepatobiliary Disorders:0.1%–<1% – cholecystitis, hepatitis; <0.1% – cholestasis.
Renal and Urinary Disorders:0.1%–<1% – renal failure, urinary frequency, proteinuria.
Neoplasms Benign, Malignant, and Unspecified:0.1%–<1% – tumor lysis syndrome.
Immune System Disorders:0.1%–<1% – hypersensitivity (including erythema nodosum).
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Dasatinib Plasma Concentrations
CYP3A4 Inhibitors: Dasatinib is a CYP3A4 substrate. In a study of 18 patients with solid tumors, 20-mg SPRYCEL once daily coadministered with 200 mg of ketoconazole twice daily increased the dasatinib Cmax and AUC by four- and five-fold, respectively. Concomitant use of SPRYCEL and drugs that inhibit CYP3A4 may increase exposure to dasatinib and should be avoided. In patients receiving treatment with SPRYCEL, close monitoring for toxicity and a SPRYCEL dose reduction should be considered if systemic administration of a potent CYP3A4 inhibitor cannot be avoided [see Dosage and Administration (2.1)].
7.2 Drugs That May Decrease Dasatinib Plasma Concentrations
CYP3A4 Inducers: When a single morning dose of SPRYCEL was administered following 8 days of continuous evening administration of 600 mg of rifampin, a potent CYP3A4 inducer, the mean Cmax and AUC of dasatinib were decreased by 81% and 82%, respectively. Alternative agents with less enzyme induction potential should be considered. If SPRYCEL must be administered with a CYP3A4 inducer, a dose increase in SPRYCEL should be considered [see Dosage and Administration (2.1)].
Antacids: Nonclinical data demonstrate that the solubility of dasatinib is pH dependent. In a study of 24 healthy subjects, administration of 30 mL of aluminum hydroxide/magnesium hydroxide 2 hours prior to a single 50-mg dose of SPRYCEL was associated with no relevant change in dasatinib AUC; however, the dasatinib Cmax increased 26%. When 30 mL of aluminum hydroxide/magnesium hydroxide was administered to the same subjects concomitantly with a 50-mg dose of SPRYCEL, a 55% reduction in dasatinib AUC and a 58% reduction in Cmax were observed. Simultaneous administration of SPRYCEL with antacids should be avoided. If antacid therapy is needed, the antacid dose should be administered at least 2 hours prior to or 2 hours after the dose of SPRYCEL.
H2 Antagonists/Proton Pump Inhibitors: Long-term suppression of gastric acid secretion by H2 antagonists or proton pump inhibitors (eg, famotidine and omeprazole) is likely to reduce dasatinib exposure. In a study of 24 healthy subjects, administration of a single 50-mg dose of SPRYCEL 10 hours following famotidine reduced the AUC and Cmax of dasatinib by 61% and 63%, respectively. The concomitant use of H2 antagonistsor proton pump inhibitors with SPRYCEL is not recommended. The use of antacids should be considered in place of H2 antagonists or proton pump inhibitors in patients receiving SPRYCEL therapy.
7.3 Drugs That May Have Their Plasma Concentration Altered By Dasatinib
CYP3A4 Substrates: Single-dose data from a study of 54 healthy subjects indicate that the mean Cmax and AUC of simvastatin, a CYP3A4 substrate, were increased by 37% and 20%, respectively, when simvastatin was administered in combination with a single 100-mg dose of SPRYCEL. Therefore, CYP3A4 substrates known to have a narrow therapeutic index such as alfentanil, astemizole, terfenadine, cisapride, cyclosporine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus, or ergot alkaloids (ergotamine, dihydroergotamine) should be administered with caution in patients receiving SPRYCEL.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.5)].
8.3 Nursing Mothers
It is unknown whether SPRYCEL is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from SPRYCEL, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and efficacy of SPRYCEL in patients <18 years of age have not been established.
8.5 Geriatric Use
Of the 2182 patients in clinical studies of SPRYCEL, 547 (25%) were 65 years of age and over, and 105 (5%) were 75 years of age and over. While the safety profile of SPRYCEL in the geriatric population was similar to that in the younger population, patients aged 65 years and older are more likely to experience fluid retention events and should be monitored closely. No differences in efficacy were observed between older and younger patients. However, in the two randomized studies in patients with chronic phase CML, the rates of major cytogenetic response (MCyR) were lower among patients aged 65 years and over.
8.6 Hepatic Impairment
There are currently no clinical studies with SPRYCEL in patients with impaired liver function (clinical studies excluded patients with ALT or AST >2.5 times the upper limit of the normal range or total bilirubin >2 times the upper limit of the normal range). Metabolism of dasatinib is mainly hepatic. Caution is recommended in patients with hepatic impairment.
8.7 Renal Impairment
There are currently no clinical studies with SPRYCEL in patients with impaired renal function. Less than 4% of dasatinib and its metabolites are excreted via the kidney.
10 OVERDOSAGE
Experience with overdose of SPRYCEL in clinical studies is limited to isolated cases. The highest reported dosage ingested was 280 mg per day for 1 week in two patients and both developed severe myelosuppression and bleeding. Since SPRYCEL is associated with severe myelosuppression [see Warnings and Precautions (5.1)and Adverse Reactions (6.1)], patients who ingested more than the recommended dosage should be closely monitored for myelosuppression and appropriate supportive treatment given.
Acute overdose in animals was associated with cardiotoxicity. Evidence of cardiotoxicity included ventricular necrosis and valvular/ventricular/atrial hemorrhage at single doses ≥100 mg/kg (600 mg/m2) in rodents. There was a tendency for increased systolic and diastolic blood pressure in monkeys at single doses ≥10 mg/kg (120 mg/m2).
11 DESCRIPTION
SPRYCEL (dasatinib) is an inhibitor of multiple tyrosine kinases. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C22H26ClN7O2S• H2O, which corresponds to a formula weight of 506.02 (monohydrate). The anhydrous free base has a molecular weight of 488.01. Dasatinib has the following chemical structure:
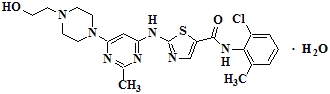
Dasatinib is a white to off-white powder. The drug substance is insoluble in water and slightly soluble in ethanol and methanol. SPRYCEL tablets are white to off-white, biconvex, film-coated tablets containing dasatinib, with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dasatinib, at nanomolar concentrations, inhibits the following kinases: BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ. Based on modeling studies, dasatinib is predicted to bind to multiple conformations of the ABL kinase.
In vitro, dasatinib was active in leukemic cell lines representing variants of imatinib mesylate sensitive and resistant disease. Dasatinib inhibited the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. Under the conditions of the assays, dasatinib was able to overcome imatinib resistance resulting from BCR-ABL kinase domain mutations, activation of alternate signaling pathways involving the SRC family kinases (LYN, HCK), and multi-drug resistance gene overexpression.
12.3 Pharmacokinetics
Absorption
Maximum plasma concentrations (Cmax) of dasatinib are observed between 0.5 and 6 hours (Tmax) following oral administration. Dasatinib exhibits dose proportional increases in AUC and linear elimination characteristics over the dose range of 15 mg to 240 mg/day. The overall mean terminal half-life of dasatinib is 3–5 hours.
Data from a study of 54 healthy subjects administered a single, 100-mg dose of dasatinib 30 minutes following consumption of a high-fat meal resulted in a 14% increase in the mean AUC of dasatinib. The observed food effects were not clinically relevant.
Distribution
In patients, dasatinib has an apparent volume of distribution of 2505 L, suggesting that the drug is extensively distributed in the extravascular space. Binding of dasatinib and its active metabolite to human plasma proteins in vitro was approximately 96% and 93%, respectively, with no concentration dependence over the range of 100–500 ng/mL.
Metabolism
Dasatinib is extensively metabolized in humans, primarily by the cytochrome P450 enzyme 3A4. CYP3A4 was the primary enzyme responsible for the formation of the active metabolite. Flavin-containing monooxygenase 3 (FMO-3) and uridine diphosphate-glucuronosyltransferase (UGT) enzymes are also involved in the formation of dasatinib metabolites. In human liver microsomes, dasatinib was a weak time-dependent inhibitor of CYP3A4.
The exposure of the active metabolite, which is equipotent to dasatinib, represents approximately 5% of the dasatinib AUC. This indicates that the active metabolite of dasatinib is unlikely to play a major role in the observed pharmacology of the drug. Dasatinib also had several other inactive oxidative metabolites.
Dasatinib is a time-dependent inhibitor of CYP3A4. At clinically relevant concentrations, dasatinib does not inhibit CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, or 2E1. Dasatinib is not an inducer of human CYP enzymes.
Elimination
Elimination is primarily via the feces. Following a single oral dose of [14C]-labeled dasatinib, approximately 4% and 85% of the administered radioactivity was recovered in the urine and feces, respectively, within 10 days. Unchanged dasatinib accounted for 0.1% and 19% of the administered dose in urine and feces, respectively, with the remainder of the dose being metabolites.
Effects of Age and Gender
Pharmacokinetic analyses of demographic data indicate that there are no clinically relevant effects of age and gender on the pharmacokinetics of dasatinib.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were not performed with dasatinib.
Dasatinib was clastogenic when tested in vitro in Chinese hamster ovary cells, with and without metabolic activation. Dasatinib was not mutagenic when tested in an in vitro bacterial cell assay (Ames test) and was not genotoxic in an in vivo rat micronucleus study.
The effects of dasatinib on male and female fertility have not been studied. However, results of repeat-dose toxicity studies in multiple species indicate the potential for dasatinib to impair reproductive function and fertility. Effects evident in male animals included reduced size and secretion of seminal vesicles, and immature prostate, seminal vesicle, and testis. The administration of dasatinib resulted in uterineinflammation and mineralization in monkeys, and cystic ovaries and ovarian hypertrophy in rodents.
14 CLINICAL STUDIES
Phase 2 Single-Arm Studies
Four Phase 2, single-arm, multicenter studies were conducted to determine the efficacy and safety of SPRYCEL in patients with CML or Ph+ ALL resistant to or intolerant of treatment with imatinib. Resistance to imatinib included failure to achieve a complete hematologic response (within 3–6 months) or major cytogenetic response (by month 12) or progression of disease after a previous cytogenetic or hematologic response. Imatinib intolerance included inability to tolerate 400 mg or more of imatinib per day or discontinuation of imatinib because of toxicity. The chronic phase CML study enrolled 186 patients, the accelerated phase CML study 107 patients, the myeloid blast phase study 74 patients, and the lymphoid blast phase CML/Ph+ ALL study 78 patients. The studies are ongoing. The results are based on a minimum of 6 months follow-up after the start of SPRYCEL therapy. Across all studies, 49% of patients were women, 89% were white, 10% were black or Asian, 23% were over the age of 65 years, and 3% were over the age of 75 years. Most patients had long disease histories with extensive prior treatment, including imatinib, cytotoxic chemotherapy, interferon, and stem cell transplant (Table 7). The maximum imatinib dose had been 400–600 mg/day in about one-half of the patients and >600 mg/day in the other half.
| Chronic (n=186) | Accelerated (n=107) | Myeloid
Blast (n=74) | Lymphoid
Blast (n=42) | Ph+
ALL (n=36) |
|
|---|---|---|---|---|---|
| Median time since diagnosis in months (range) | 64 (4–251) | 91 (4–355) | 49 (3–216) | 28 (2–186) | 20 (3–97) |
| Imatinib | |||||
| Resistant | 68% | 93% | 92% | 88% | 94% |
| Intolerant | 32% | 7% | 8% | 12% | 6% |
| Imatinib | |||||
| >3 years | 54% | 68% | 47% | 24% | 3% |
| >1 year | 80% | 92% | 85% | 52% | 56% |
| Cytotoxic chemotherapy | 42% | 67% | 66% | 79% | 92% |
| Interferon | 70% | 75% | 55% | 48% | 8% |
| Stem cell transplant | 9% | 18% | 12% | 33% | 42% |
All patients were treated with SPRYCEL 70 mg twice daily on a continuous basis. The median durations of treatment are shown in Table 8.
| Chronic (n=186) | Accelerated (n=107) | Myeloid
Blast (n=74) | Lymphoid
Blast (n=42) | Ph+
ALL (n=36) |
|
|---|---|---|---|---|---|
| Median duration of therapy in months (range) | 5.6
(0.03–8.3) | 5.5
(0.2–10.1) | 3.5
(0.03–9.2) | 2.8
(0.1–6.4) | 3.2
(0.2–8.1) |
The primary efficacy endpoint in chronic phase CML was major cytogenetic response (MCyR), defined as elimination (complete cytogenetic response, CCyR) or substantial diminution (by at least 65%, partial cytogenetic response) of Ph+ hematopoietic cells. The primary endpoint in accelerated phase, myeloid blast phase, and lymphoid blast phase CML, and Ph+ ALL was major hematologic response (MaHR), defined as either a complete hematologic response (CHR) or no evidence of leukemia (NEL) (defined in Table 9).
SPRYCEL treatment resulted in cytogenetic and hematologic responses in patients with all phases of CML and with Ph+ ALL. The response rates for the single-arm studies are reported in Table 9. In chronic phase CML patients, the MCyR rate was 45% with a complete response (0% Ph+ cells) rate of 33%. The MaHR rate was 59% in accelerated phase patients, 32% in myeloid phase patients, 31% in lymphoid blast phase patients, and 42% in Ph+ ALL patients.
Most cytogenetic responses occurred after 12 weeks of treatment, when the first cytogenetic analyses were performed. Hematologic and cytogenetic responses were stable during the 6-month follow-up of patients with chronic phase, accelerated phase, and myeloid blast phase CML. The median durations of MaHR were 3.7 months in lymphoid blast CML and 4.8 months in Ph+ ALL.
There were no age- or gender-related response differences.
| Chronic (n=186) | Accelerated (n=107) | Myeloid
Blast (n=74) | Lymphoid
Blast (n=42) | Ph+
ALL (n=36) |
|
|---|---|---|---|---|---|
| a Numbers in bold font are the results of primary endpoints. | |||||
| b Hematologic
response criteria (all responses confirmed after 4 weeks): Major hematologic response: (MaHR) = complete hematologic response (CHR) + no evidence of leukemia (NEL). CHR (chronic CML): WBC ≤ institutional ULN, platelets <450,000/mm3, no blasts or promyelocytes in peripheral blood, <5% myelocytes plus metamyelocytes in peripheral blood, basophils in peripheral blood <20%, and no extramedullary involvement. CHR (advanced CML/Ph+ ALL): WBC ≤ institutional ULN, ANC ≥1000/mm3, platelets≥100,000/mm3, no blasts or promyelocytes in peripheral blood, bone marrow blasts ≤5%, <5% myelocytes plus metamyelocytes in peripheral blood, basophils in peripheral blood <20%, and no extramedullary involvement. NEL: same criteria as for CHR but ANC ≥500/mm3 and <1000/mm3, or platelets ≥20,000/mm3 and ≤100,000/mm3. |
|||||
| c Cytogenetic response criteria: complete (0% Ph+ metaphases) or partial (>0%–35%). MCyR (0%–35%) combines both complete and partial responses. | |||||
| n/a = not applicable. | |||||
| Hematologic Response Rateb (%) | |||||
| MaHR (95% CI) | n/a | 59 (49–68) | 32 (22–44) | 31 (18–47) | 42 (26–59) |
| CHR (95% CI) | 90 (85–94) | 33 (24–42) | 24 (15–36) | 26 (14–42) | 31 (16–48) |
| NEL (95% CI) | n/a | 26 (18–36) | 8 (3–17) | 5 (0.6–16) | 11 (3.1–26) |
| Cytogenetic Responsec (%) | |||||
| MCyR (95% CI) | 45 (37–52) | 31 (22–41) | 30 (20–42) | 50 (34–66) | 58 (41–74) |
| CCyR (95% CI) | 33 (26–40) | 21 (14–30) | 27 (17–39) | 43 (28–59) | 58 (41–74) |
Randomized Studies
Phase 2 randomized study of SPRYCEL 70 mg twice daily or imatinib 800 mg daily (400 mg twice daily). A randomized, open-label study was conducted in patients with chronic phase CML whose disease was resistant to prior imatinib therapy at doses of 400 or 600 mg. The primary endpoint was MCyR at 12 weeks. One hundred fifty patients were randomized in a 2:1 ratio to either SPRYCEL 70 mg twice daily or imatinib 800 mg daily (400 mg twice daily). Crossover to the alternate therapy was permitted in the event of disease progression or intolerable toxicity. Median follow-up was 15 months. Median duration of treatment prior to crossover was 14 months for SPRYCEL and 3 months for imatinib.
Prior to crossover, 93% of the SPRYCEL-treated patients and 82% of the imatinib-treated patients achieved a CHR. At 12 weeks, MCyR was achieved in 36% of the SPRYCEL-treated patients (CCyR in 22%) and 29% of the imatinib-treated patients (CCyR in 8%). With longer treatment and follow-up, MCyR was achieved in 52% of the SPRYCEL-treated patients (CCyR in 40%) and 33% of the imatinib-treated patients (CCyR in 16%) prior to crossover. Since the median follow-up was 15 months, there were too few progressions to reliably estimate the duration of MCyR.
Phase 3 dose-optimization study in chronic phase CML: A randomized, open-label study was conducted in patients with chronic phase CML, whose disease was resistant to or who were intolerant to imatinib, to evaluate the efficacy of SPRYCEL administered once daily compared with SPRYCEL administered twice daily. Patients with significant cardiac diseases including myocardial infarction within 6 months, congestive heart failure within 3 months, significant arrhythmias, or QTc prolongation were excluded from the study. The primary endpoint was MCyR in patients with imatinib-resistant chronic phase CML. The main secondary endpoint was MCyR by total daily dose level in the same population. A total of 670 patients, of whom 498 had imatinib resistant disease, were randomized to the SPRYCEL 100 mg once daily, 140 mg once daily, 50 mg twice daily, or 70 mg twice daily group. Minimum follow-up was 6 months and median duration of treatment was approximately 8 months.
Response rates are presented in Table 10. Efficacy was achieved across all SPRYCEL treatment groups with the once daily schedule demonstrating comparable efficacy (non-inferiority) to the twice daily schedule on the primary efficacy endpoint (difference in MCyR 2.8%; 95% confidence interval [-6.0%–11.6%]). The main secondary endpoint of the study also showed comparable efficacy (non-inferiority) between the 100 mg total daily dose and the 140 mg total daily dose (difference in MCyR -0.8%; 95% confidence interval [-9.6%–8.0%]). Since the minimum follow-up was only 6 months, there were too few progressions to estimate the duration of MCyR.
| 100
mg QD
(N=167) | 50 mg BIDa
(N=168) | 140 mg QDa
(N=167) | 70 mg BIDa
(N=168) |
|
|---|---|---|---|---|
| a Not a recommended starting dosage of SPRYCEL for chronic phase CML. | ||||
| b Hematologic
response criteria (confirmed after 4 weeks): CHR (chronic CML): WBC ≤ institutional ULN, platelets <450,000/mm3, no blasts or promyelocytes in peripheral blood, <5% myelocytes plus metamyelocytes in peripheral blood, basophils in peripheral blood <20%, and no extramedullary involvement. |
||||
| c Cytogenetic response criteria: complete (0% Ph+ metaphases) or partial (>0%–35%). MCyR (0%–35%) combines both complete and partial responses. | ||||
| Hematologic Response Rateb (%) | ||||
| CHR | 90% | 92% | 86% | 87% |
| Cytogenetic Responsec (%) | ||||
| MCyR | ||||
| All patients (95% CI) | 59% (51–66) | 54% (46–61) | 56% (48–63) | 55% (48–63) |
| Imatinib-resistant patients (95%
CI) (n/N) | 53% (44–62) (66/124) | 47% (38–56) (58/124) | 50% (41–60) (62/123) | 51% (42–60) (65/127) |
| CCyR | ||||
| All patients | 41% | 42% | 44% | 45% |
| Imatinib-resistant patients (95%
CI) (n/N) | 34% (26–43) (42/124) | 35% (26–44) (43/124) | 36% (27–45) (44/123) | 39% (31–48) (50/127) |
15 REFERENCES
- Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH Alert 2004–165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999, http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html.
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. (2006) 63:1172–1193.
- Polovich M, White JM, Kelleher LO (eds). 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd ed). Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SPRYCEL® (dasatinib) tablets are available as described in Table 11.
| NDC Number | Strength | Description | Tablets per Bottle |
| 0003-0527-11 | 20 mg | white to off-white, biconvex, round, film-coated tablet with “BMS” debossed on one side and “527” on the other side | 60 |
| 0003-0528-11 | 50 mg | white to off-white, biconvex, oval, film-coated tablet with “BMS” debossed on one side and “528” on the other side | 60 |
| 0003-0524-11 | 70 mg | white to off-white, biconvex, round, film-coated tablet with “BMS” debossed on one side and “524” on the other side | 60 |
| 0003-0852–22 | 100 mg | white to off-white, biconvex, oval, film-coated tablet with “BMS 100” debossed on one side and “852” on the other side | 30 |
16.2 Storage
SPRYCEL tablets should be stored at 25° C (77° F); excursions permitted between 15°–30° C (59°–86° F) [see USP Controlled Room Temperature].
16.3 Handling and Disposal
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published [see References (15)].
SPRYCEL (dasatinib) tablets consist of a core tablet (containing the active drug substance), surrounded by a film coating to prevent exposure of pharmacy and clinical personnel to the active drug substance. However, if tablets are inadvertently crushed or broken, pharmacy and clinical personnel should wear disposable chemotherapy gloves. Personnel who are pregnant should avoid exposure to crushed or broken tablets.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.10).
17.1 Bleeding
Patients should be informed of the possibility of serious bleeding and to report immediately any signs or symptoms suggestive of hemorrhage (unusual bleeding or easy bruising).
17.2 Myelosuppression
Patients should be informed of the possibility of developing low blood cell counts; they should be instructed to report immediately should fever develop, particularly in association with any suggestion of infection.
17.3 Fluid Retention
Patients should be informed of the possibility of developing fluid retention (swelling, weight gain, or shortness of breath) and to seek medical attention if those symptoms arise.
17.4 Pregnancy
Patients should be informed that dasatinib may cause fetal harm when administered to a pregnant woman. Women should be advised of the potential hazard to the fetus and to avoid becoming pregnant. If SPRYCEL is used during pregnancy, or if the patient becomes pregnant while taking SPRYCEL, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.5)].
17.5 Gastrointestinal Complaints
Patients should be informed that they may experience nausea, vomiting, or diarrhea with SPRYCEL. If these symptoms are significant, they should seek medical attention.
17.6 Pain
Patients should be informed that they may experience headache or musculoskeletal pain with SPRYCEL. If these symptoms are significant, they should seek medical attention.
17.7 Fatigue
Patients should be informed that they may experience fatigue with SPRYCEL. If this symptom is significant, they should seek medical attention.
17.8 Rash
Patients should be informed that they may experience skin rash with SPRYCEL. If this symptom is significant, they should seek medical attention.
17.9 Lactose
Patients should be informed that SPRYCEL contains 135 mg of lactose monohydrate in a 100-mg daily dose and 189 mg of lactose monohydrate in a 140-mg daily dose.
Manufactured by:
Bristol-Myers Squibb Company
Princeton,
NJ 08543 USA
US Patent No 6,596,746
Bristol-Myers Squibb
Princeton,
NJ 08543 USA
1237674A3
Rev
October 2008
17.10 FDA-Approved Patient Labeling
SPRYCEL® (dasatinib) Tablets
What is SPRYCEL?
SPRYCEL (dasatinib) is a prescription medicine used to treat adults who have chronic myeloid leukemia (CML) and to treat adults who have a particular form of acute lymphoblastic leukemia (ALL) called Philadelphia chromosome positive or Ph+ ALL. It is intended for use in patients who are no longer benefiting from treatment with the current available therapiesfor these diseases (resistance), including a medicine called GLEEVEC® (imatinib mesylate). It may also be used in patients who experience severe side effects from GLEEVEC and are no longer able to take it (intolerance). The long-term benefits and toxicities of SPRYCEL are currently still being studied. SPRYCEL has not been studied in children.
What is Leukemia?
Leukemia is a cancer of white blood cells, which grow in the bone marrow. In leukemia, white blood cells multiply in an uncontrolled manner, occupying the bone marrow space and spilling out into the bloodstream. As a consequence, the production of normal red blood cells (oxygen carrying cells), white blood cells (cells which fight infection), and platelets (cells which help blood clot) is compromised. Therefore, patients with leukemia are at risk of serious anemia, infections, and bleeding.
Chronic myeloid leukemia or CML is one form of leukemia. In CML, myeloid white blood cells multiply in an uncontrolled manner. It may take years for CML to progress because it is a slow-growing or chronic cancer. As CML progresses, patients advance through three phases: chronic phase, accelerated phase, and blast crisis phase. Ph+ acute lymphoblastic leukemia or Ph+ ALL is another form of leukemia. Acute leukemias progress faster than chronic leukemias. In Ph+ ALL, lymphoblastic white blood cells multiply in an uncontrolled manner.
How does SPRYCEL work?
The active ingredient of SPRYCEL is dasatinib. Dasatinib reduces the activity of one or more proteins responsible for the uncontrolled growth of the leukemia cells of patients with CML or Ph+ ALL. This reduction allows the bone marrow to resume production of normal red cells, white cells, and platelets.
Who should not take SPRYCEL?
- SPRYCEL is currently not recommended for patients who have not previously had a trial of GLEEVEC® (imatinib mesylate).
- Women who are pregnant or planning to become pregnant should not take SPRYCEL (see below).
What should I tell my healthcare provider before I take SPRYCEL?
Tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or planning to become pregnant. SPRYCEL may harm the fetus when given to a pregnant woman. Women should avoid becoming pregnant while undergoing treatment with SPRYCEL. Tell your healthcare provider immediately if you become pregnant or plan to become pregnant while taking SPRYCEL.
- are breast-feeding. It is not known if SPRYCEL can pass into your breast milk or if it can harm your baby. Do not breast-feed if you are taking SPRYCEL.
- are a sexually active male. Men who take SPRYCEL are advised to use a condom to avoid pregnancy in their partner.
- have a liver or heart problem.
- are lactose intolerant.
Can I take other medicines with SPRYCEL?
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, antacids, and herbal supplements.
SPRYCEL is eliminated from your body through the liver. The use of certain other medicines may alter the levels of SPRYCEL in your bloodstream. Likewise, levels of other medicines in your bloodstream can be affected by SPRYCEL. Such changes can increase the side effects, or reduce the activity of the medicines you are taking, including SPRYCEL.
- Medicines that increase the amount of SPRYCEL in your bloodstream are NIZORAL® (ketoconazole), SPORANOX® (itraconazole), NORVIR® (ritonavir), REYATAZ® (atazanavir sulfate), CRIXIVAN® (indinavir), VIRACEPT® (nelfinavir), INVIRASE® (saquinavir), KETEK® (telithromycin), E-MYCIN® (erythromycin), and BIAXIN® (clarithromycin).
- Medicines that decrease the amount of SPRYCEL in your bloodstream are DECADRON® (dexamethasone), DILANTIN® (phenytoin), TEGRETOL® (carbamazepine), RIMACTANE® (rifampin), and LUMINAL® (phenobarbital).
- Medicines whose blood levels might be altered by SPRYCEL are SANDIMMUNE® (cyclosporine), ALFENTA® (alfentanil), FENTANYL® (fentanyl), ORAP® (pimozide), RAPAMUNE® (sirolimus), PROGRAF® (tacrolimus), and ERGOMAR® (ergotamine).
SPRYCEL is best absorbed from your stomach into your bloodstream in the presence of stomach acid. You should avoid taking medicines that reduce stomach acid such as TAGAMET® (cimetidine), PEPCID® (famotidine), ZANTAC® (ranitidine), PRILOSEC® (omeprazole), PROTONIX® (pantoprazole sodium), NEXIUM® (esomeprazole), ACIPHEX® (rabeprazole), or PREVACID® (lansoprazole) while taking SPRYCEL. Medicines that neutralize stomach acid, such as MAALOX® (aluminum hydroxide/magnesium hydroxide), TUMS® (calcium carbonate), or ROLAIDS® (calcium carbonate and magnesia) may be taken up to 2 hours before or 2 hours after SPRYCEL.
Since SPRYCEL therapy may cause bleeding, tell your healthcare provider if you are using blood thinners, such as COUMADIN® (warfarin sodium) or aspirin.
How should I take SPRYCEL?
- If you have chronic phase CML, the usual dose is 100 mg (one 100-mg tablet or two 50-mg tablets) once daily, either in the morning or in the evening.
- If you have accelerated or blast crisis CML or Ph+ ALL, the usual dose is 70 mg (one 70-mg tablet) twice daily, once in the morning and once in the evening.
- SPRYCEL can be taken with or without a meal. Try to take SPRYCEL at the same time each day.
- Take SPRYCEL whole. Do not break, cut, or crush the tablets.
- Do not drink grapefruit juice while taking SPRYCEL.
- Depending on your response to treatment and any side effects that you may experience, your healthcare provider may adjust your dose of SPRYCEL upward or downward, or may temporarily discontinue SPRYCEL.
- You should not change your dose or stop taking SPRYCEL without first talking with your healthcare provider.
- If you miss a dose of SPRYCEL, take your next scheduled dose at its regular time. Do not take two doses at the same time. Call your healthcare provider or pharmacist if you are not sure what to do.
- If you accidentally take more than the prescribed dose of SPRYCEL, call your healthcare provider right away.
What are the possible side effects of SPRYCEL?
The following information describes the most important side effects of SPRYCEL. It is not a comprehensive list of all side effects recorded in clinical trials with SPRYCEL. You should report any unusual symptoms to your healthcare provider.
- Low Blood Counts: SPRYCEL may cause low red blood cell counts (anemia), low white blood cell counts (neutropenia), and low platelet counts (thrombocytopenia). Your healthcare provider will monitor your blood counts frequently after you start SPRYCEL and may adjust your dose of SPRYCEL or withhold the drug temporarily in the event your blood counts drop too low. In some cases, you may need to receive transfusions of red blood cells or platelets. Notify your healthcare provider immediately if you develop a fever while taking SPRYCEL.
- Bleeding: SPRYCEL may cause bleeding. The most serious bleeding events observed in clinical studies included bleeding into the brain leading to death in <1% of patients, and bleeding from the gastrointestinal tract. Less severe events included bleeding from the nose, the gums, bruising of the skin, and excessive menstrual bleeding. Notify your healthcare provider immediately if you experience bleeding or easy bruising while taking SPRYCEL.
- Fluid Retention: SPRYCEL may cause fluid to accumulate in your legs and around your eyes. In more severe cases, fluid may accumulate in the lining of your lungs, the sac around your heart, or your abdominal cavity. Notify your healthcare provider immediately if you experience swelling, weight gain, or increasing shortness of breath while taking SPRYCEL.
Other common side effects of SPRYCEL therapy include diarrhea, headache, skin rash, nausea, fatigue, and shortness of breath.
In clinical trials of 2182 patients, 10% (10 out of 100) of patients permanently stopped SPRYCEL therapy because of side effects.
How will I know if SPRYCEL is working?
How well you respond to SPRYCEL therapy may depend on several factors, including the phase of your disease, prior treatments, or other factors your healthcare provider may discuss with you. General treatment goals for patients treated with SPRYCEL include a reduction in the number of leukemia cells and improvement or normalization of the white blood cell, red blood cell, and platelet counts.
While you are on SPRYCEL, your healthcare provider will monitor these responses through routine blood tests. The type and frequency of these tests will be determined by your healthcare provider and may vary depending on the status of your disease.
How should I store SPRYCEL?
- Store SPRYCEL (dasatinib) Tablets at room temperature, 59° to 86° F (15° to 30° C). SPRYCEL Tablets do not require refrigeration.
- Keep the container tightly closed.
- Throw away SPRYCEL when it is outdated. Ask your pharmacist how to properly dispose of SPRYCEL.
- Keep SPRYCEL and all medicines out of the reach of children and pets.
General information about SPRYCEL: This medicine was prescribed for your particular condition and should be used only by you under the close supervision of your healthcare provider. The leaflet summarizes the most important information about SPRYCEL. If you would like more information, talk with your healthcare provider. If you have questions or concerns, or want more information about SPRYCEL, your healthcare provider and pharmacist have the complete prescribing information upon which this guide is based. You may want to read it and discuss it with your healthcare provider. Remember, no written summary can replace careful discussion with your healthcare provider.
What are the ingredients in SPRYCEL?
Active Ingredient: dasatinib
Inactive Ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
——————————————
REYATAZ® is a registered trademark of Bristol-Myers
Squibb Company. COUMADIN® is a registered trademark
of Bristol-Myers Squibb Pharma Company. Other brands listed are the trademarks
of their respective owners and are not trademarks of Bristol-Myers Squibb
Company.
Manufactured by:
Bristol-Myers Squibb Company
Princeton,
NJ 08543 USA
US Patent No 6,596,746
Bristol-Myers Squibb
Princeton,
NJ 08543 USA
1237674A3
Rev
October 2008
What are the ingredients in SPRYCEL?
What are the ingredients in SPRYCEL?
Active Ingredient: dasatinib
Inactive Ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The tablet coating consists of hypromellose, titanium dioxide, and polyethylene glycol.
——————————————
REYATAZ® is a registered trademark of Bristol-Myers
Squibb Company. COUMADIN® is a registered trademark
of Bristol-Myers Squibb Pharma Company. Other brands listed are the trademarks
of their respective owners and are not trademarks of Bristol-Myers Squibb
Company.
Manufactured by:
Bristol-Myers Squibb Company
Princeton,
NJ 08543 USA
US Patent No 6,596,746
Bristol-Myers Squibb
Princeton,
NJ 08543 USA
1237674A3
Rev
October 2008
| SPRYCEL
dasatinib tablet |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| SPRYCEL
dasatinib tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| SPRYCEL
dasatinib tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| SPRYCEL
dasatinib tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
Revised: 10/2008Bristol-Myers Squibb