remicade (infliximab) injection, powder, lyophilized, for solution
[Centocor, Inc.]
WARNINGS
RISK OF INFECTIONS
Patients treated with REMICADE are at increased risk for infections, including progression to serious infections leading to hospitalization or death (see WARNINGS and ADVERSE REACTIONS). These infections have included bacterial sepsis, tuberculosis, invasive fungal and other opportunistic infections. Patients should be educated about the symptoms of infection, closely monitored for signs and symptoms of infection during and after treatment with REMICADE, and should have access to appropriate medical care. Patients who develop an infection should be evaluated for appropriate antimicrobial therapy and for serious infections REMICADE should be discontinued.
Tuberculosis (frequently disseminated or extrapulmonary at clinical presentation) has been observed in patients receiving REMICADE. Patients should be evaluated for tuberculosis risk factors and be tested for latent tuberculosis infection1, 2prior to initiating REMICADE and during therapy. Treatment of latent tuberculosis infection should be initiated prior to therapy with REMICADE. Treatment of latent tuberculosis in patients with a reactive tuberculin test reduces the risk of tuberculosis reactivation in patients receiving REMICADE. Some patients who tested negative for latent tuberculosis prior to receiving REMICADE have developed active tuberculosis. Physicians should monitor patients receiving REMICADE for signs and symptoms of active tuberculosis, including patients who tested negative for latent tuberculosis infection.
HEPATOSPLENIC T-CELL LYMPHOMAS
Rare postmarketing cases of hepatosplenic T-cell lymphoma have been reported in adolescent and young adult patients with Crohn's disease treated with REMICADE. This rare type of T-cell lymphoma has a very aggressive disease course and is usually fatal. All of these hepatosplenic T-cell lymphomas with REMICADE have occurred in patients on concomitant treatment with azathioprine or 6-mercaptopurine.
DESCRIPTION
REMICADE is a chimeric IgG1κ monoclonal antibody with an approximate molecular weight of 149,100 daltons. It is composed of human constant and murine variable regions. Infliximab binds specifically to human tumor necrosis factor alpha (TNFα) with an association constant of 1010 M-1. Infliximab is produced by a recombinant cell line cultured by continuous perfusion and is purified by a series of steps that includes measures to inactivate and remove viruses.
REMICADE is supplied as a sterile, white, lyophilized powder for intravenous infusion. Following reconstitution with 10 mL of Sterile Water for Injection, USP, the resulting pH is approximately 7.2. Each single-use vial contains 100 mg infliximab, 500 mg sucrose, 0.5 mg polysorbate 80, 2.2 mg monobasic sodium phosphate, monohydrate, and 6.1 mg dibasic sodium phosphate, dihydrate. No preservatives are present.
CLINICAL PHARMACOLOGY
General
Infliximab neutralizes the biological activity of TNFα by binding with high affinity to the soluble and transmembrane forms of TNFα and inhibits binding of TNFα with its receptors.3,4 Infliximab does not neutralize TNFβ (lymphotoxin α), a related cytokine that utilizes the same receptors as TNFα. Biological activities attributed to TNFα include: induction of pro-inflammatory cytokines such as interleukins (IL) 1 and 6, enhancement of leukocyte migration by increasing endothelial layer permeability and expression of adhesion molecules by endothelial cells and leukocytes, activation of neutrophil and eosinophil functional activity, induction of acute phase reactants and other liver proteins, as well as tissue degrading enzymes produced by synoviocytes and/or chondrocytes. Cells expressing transmembrane TNFα bound by infliximab can be lysed in vitro4 or in vivo.5 Infliximab inhibits the functional activity of TNFα in a wide variety of in vitro bioassays utilizing human fibroblasts, endothelial cells, neutrophils, B and T lymphocytes and epithelial cells. The relationship of these biological response markers to the mechanism(s) by which REMICADE exerts its clinical effects is unknown. Anti-TNFα antibodies reduce disease activity in the cotton-top tamarin colitis model, and decrease synovitis and joint erosions in a murine model of collagen-induced arthritis. Infliximab prevents disease in transgenic mice that develop polyarthritis as a result of constitutive expression of human TNFα, and when administered after disease onset, allows eroded joints to heal.
Pharmacodynamics
Elevated concentrations of TNFα have been found in involved tissues and fluids of patients with rheumatoid arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis. In rheumatoid arthritis, treatment with REMICADE reduced infiltration of inflammatory cells into inflamed areas of the joint as well as expression of molecules mediating cellular adhesion [E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], chemoattraction [IL-8 and monocyte chemotactic protein (MCP-1)] and tissue degradation [matrix metalloproteinase (MMP) 1 and 3]. In Crohn's disease, treatment with REMICADE reduced infiltration of inflammatory cells and TNFα production in inflamed areas of the intestine, and reduced the proportion of mononuclear cells from the lamina propria able to express TNFα and interferon. After treatment with REMICADE, patients with rheumatoid arthritis or Crohn's disease exhibited decreased levels of serum IL-6 and C-reactive protein (CRP) compared to baseline. Peripheral blood lymphocytes from REMICADE-treated patients showed no significant decrease in number or in proliferative responses to in vitro mitogenic stimulation when compared to cells from untreated patients. In psoriatic arthritis, treatment with REMICADE resulted in a reduction in the number of T-cells and blood vessels in the synovium and psoriatic skin lesions as well as a reduction of macrophages in the synovium. In plaque psoriasis, REMICADE treatment may reduce the epidermal thickness and infiltration of inflammatory cells. The relationship between these pharmacodynamic activities and the mechanism(s) by which REMICADE exerts its clinical effects is unknown.
Pharmacokinetics
In adults, single intravenous (IV) infusions of 3 mg/kg to 20 mg/kg showed a linear relationship between the dose administered and the maximum serum concentration. The volume of distribution at steady state was independent of dose and indicated that infliximab was distributed primarily within the vascular compartment. Pharmacokinetic results for single doses of 3 mg/kg to 10 mg/kg in rheumatoid arthritis, 5 mg/kg in Crohn's disease, and 3 mg/kg to 5 mg/kg in plaque psoriasis indicate that the median terminal half-life of infliximab is 7.7 to 9.5 days.
Following an initial dose of REMICADE, repeated infusions at 2 and 6 weeks resulted in predictable concentration-time profiles following each treatment. No systemic accumulation of infliximab occurred upon continued repeated treatment with 3 mg/kg or 10 mg/kg at 4- or 8-week intervals. Development of antibodies to infliximab increased infliximab clearance. At 8 weeks after a maintenance dose of 3 to 10 mg/kg of REMICADE, median infliximab serum concentrations ranged from approximately 0.5 to 6 mcg/mL; however, infliximab concentrations were not detectable (<0.1 mcg/mL) in patients who became positive for antibodies to infliximab. No major differences in clearance or volume of distribution were observed in patient subgroups defined by age, weight, or gender. It is not known if there are differences in clearance or volume of distribution in patients with marked impairment of hepatic or renal function.
Infliximab peak and trough concentrations were similar in pediatric (aged 6 to 17 years old) and adult patients with Crohn's disease following the administration of the recommended regimen (see DOSAGE AND ADMINISTRATION, Crohn's Disease or Fistulizing Crohn's Disease).
Population pharmacokinetic analysis showed that in children with juvenile rheumatoid arthritis (JRA) with a body weight of up to 35 kg receiving 6 mg/kg REMICADE and children with JRA with body weight greater than 35 kg up to adult body weight receiving 3mg/kg REMICADE, the steady state area under the concentration curve (AUCss) was similar to that observed in adults receiving 3 mg/kg of REMICADE.
CLINICAL STUDIES
Rheumatoid Arthritis
The safety and efficacy of REMICADE were assessed in two multicenter, randomized, double-blind, pivotal trials: ATTRACT (Study RA I) and ASPIRE (Study RA II). Concurrent use of stable doses of folic acid, oral corticosteroids (≤10 mg/day) and/or non-steroidal anti-inflammatory drugs was permitted.
Study RA I was a placebo-controlled study of 428 patients with active rheumatoid arthritis despite treatment with MTX. Patients enrolled had a median age of 54 years, median disease duration of 8.4 years, median swollen and tender joint count of 20 and 31 respectively, and were on a median dose of 15 mg/wk of MTX. Patients received either placebo + MTX or one of 4 doses/schedules of REMICADE + MTX: 3 mg/kg or 10 mg/kg of REMICADE by IV infusion at weeks 0, 2 and 6 followed by additional infusions every 4 or 8 weeks in combination with MTX.
Study RA II was a placebo-controlled study of three active treatment arms in 1004 MTX naive patients of 3 or fewer years duration active rheumatoid arthritis. Patients enrolled had a median age of 51 years with a median disease duration of 0.6 years, median swollen and tender joint count of 19 and 31, respectively, and >80% of patients had baseline joint erosions. At randomization, all patients received MTX (optimized to 20 mg/wk by week 8) and either placebo, 3mg/kg or 6 mg/kg REMICADE at weeks 0, 2, and 6 and every 8 weeks thereafter.
Data on use of REMICADE without concurrent MTX are limited (see ADVERSE REACTIONS, Immunogenicity). 6,7
Clinical response
In Study RA I, all doses/schedules of REMICADE + MTX resulted in improvement in signs and symptoms as measured by the American College of Rheumatology response criteria (ACR 20) with a higher percentage of patients achieving an ACR 20, 50 and 70 compared to placebo + MTX (Table 1). This improvement was observed at week 2 and maintained through week 102. Greater effects on each component of the ACR 20 were observed in all patients treated with REMICADE + MTX compared to placebo + MTX (Table 2). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1).
In Study RA II, after 54 weeks of treatment, both doses of REMICADE + MTX resulted in statistically significantly greater response in signs and symptoms compared to MTX alone as measured by the proportion of patients achieving ACR 20, 50 and 70 responses (Table 1). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1).
| Study RA I | Study RA II | |||||||||||||||||
| REMICADE + MTX | REMICADE + MTX | |||||||||||||||||
| 3 mg/kg | 10 mg/kg | 3 mg/kg | 6 mg/kg | |||||||||||||||
| Response | Placebo + MTX | q 8 wks | q 4 wks | q 8 wks | q 4 wks | Placebo + MTX | q 8 wks | q 8 wks |
||||||||||
| (n=88) | (n=86) | (n=86) | (n=87) | (n=81) | (n=274) | (n=351) | (n=355) | |||||||||||
|
# A major clinical response was defined as a 70% ACR response for 6 consecutive months (consecutive visits spanning at least 26 weeks) through week 102 for Study RA I and week 54 for Study RA II. |
||||||||||||||||||
|
ap ≤ 0.001 |
||||||||||||||||||
|
bp < 0.01 |
||||||||||||||||||
|
cp < 0.05 |
||||||||||||||||||
| ACR 20 | ||||||||||||||||||
| Week 30 | 20% | 50%a | 50%a | 52%a | 58%a | N/A | N/A | N/A | ||||||||||
| Week 54 | 17% | 42%a | 48%a | 59%a | 59%a | 54% | 62%c | 66%a | ||||||||||
| ACR 50 | ||||||||||||||||||
| Week 30 | 5% | 27%a | 29%a | 31%a | 26%a | N/A | N/A | N/A | ||||||||||
| Week 54 | 9% | 21%c | 34%a | 40%a | 38%a | 32% | 46%a | 50%a | ||||||||||
| ACR 70 | ||||||||||||||||||
| Week 30 | 0% | 8%b | 11%b | 18%a | 11%a | N/A | N/A | N/A | ||||||||||
| Week 54 | 2% | 11%c | 18%a | 26%a | 19%a | 21% | 33%b | 37%a | ||||||||||
| Major clinical response# | 0% | 7% c | 8% b | 15% a | 6% c | 8% | 12% | 17% a | ||||||||||
| Placebo + MTX | REMICADE + MTXa | ||||
| Parameter (medians) | (n=88) | (n=340) | |||
| Baseline | Week 54 | Baseline | Week 54 | ||
|
aAll doses/schedules of REMICADE + MTX |
|||||
|
bVisual Analog Scale (0=best, 10=worst) |
|||||
|
cHealth Assessment Questionnaire, measurement of 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (0=best, 3=worst) |
|||||
| No. of Tender Joints | 24 | 16 | 32 | 8 | |
| No. of Swollen Joints | 19 | 13 | 20 | 7 | |
| Painb | 6.7 | 6.1 | 6.8 | 3.3 | |
| Physician's Global Assessmentb | 6.5 | 5.2 | 6.2 | 2.1 | |
| Patient's Global Assessmentb | 6.2 | 6.2 | 6.3 | 3.2 | |
| Disability Index (HAQ-DI)c | 1.8 | 1.5 | 1.8 | 1.3 | |
| CRP (mg/dL) | 3.0 | 2.3 | 2.4 | 0.6 | |
Radiographic response
Structural damage in both hands and feet was assessed radiographically at week 54 by the change from baseline in the van der Heijde-modified Sharp (vdH-S) score, a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in hands/wrists and feet.8
In Study RA I, approximately 80% of patients had paired x-ray data at 54 weeks and approximately 70% at 102 weeks. The inhibition of progression of structural damage was observed at 54 weeks (Table 3) and maintained through 102 weeks.
In Study RA II, >90% of patients had at least two evaluable x-rays. Inhibition of progression of structural damage was observed at weeks 30 and 54 (Table 3) in the REMICADE + MTX groups compared to MTX alone. Patients treated with REMICADE + MTX demonstrated less progression of structural damage compared to MTX alone, whether baseline acute phase reactants (ESR and CRP) were normal or elevated: patients with elevated baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 4.2 units compared to patients treated with REMICADE + MTX who demonstrated 0.5 units of progression; patients with normal baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 1.8 units compared to REMICADE + MTX who demonstrated 0.2 units of progression. Of patients receiving REMICADE + MTX, 59% had no progression (vdH-S score ≤ 0 unit) of structural damage compared to 45% patients receiving MTX alone. In a subset of patients who began the study without erosions, REMICADE + MTX maintained an erosion free state at 1 year in a greater proportion of patients than MTX alone, 79% (77/98) vs. 58% (23/40), respectively (p<0.01). Fewer patients in the REMICADE + MTX groups (47%) developed erosions in uninvolved joints compared to MTX alone (59%).
| Study RA I | Study RA II | ||||||
| REMICADE + MTX | REMICADE + MTX | ||||||
| 3 mg/kg | 10 mg/kg | 3 mg/kg | 6 mg/kg | ||||
| Placebo + MTX | q 8 wks | q 8 wks | Placebo + MTX | q 8 wks | q 8 wks |
||
| (n=64) | (n=71) | (n=77) | (n=282) | (n=359) | (n=363) | ||
|
a P <0.001 for each outcome against placebo. |
|||||||
| Total Score | |||||||
| Baseline | |||||||
| Mean | 79 | 78 | 65 | 11.3 | 11.6 | 11.2 | |
| Median | 55 | 57 | 56 | 5.1 | 5.2 | 5.3 | |
| Change from baseline | |||||||
| Mean | 6.9 | 1.3a | 0.2a | 3.7 | 0.4a | 0.5a | |
| Median | 4.0 | 0.5 | 0.5 | 0.4 | 0.0 | 0.0 | |
|
Erosion Score | |||||||
| Baseline | |||||||
| Mean | 44 | 44 | 33 | 8.3 | 8.8 | 8.3 | |
| Median | 25 | 29 | 22 | 3.0 | 3.8 | 3.8 | |
| Change from baseline | |||||||
| Mean | 4.1 | 0.2a | 0.2a | 3.0 | 0.3a | 0.1a | |
| Median | 2.0 | 0.0 | 0.5 | 0.3 | 0.0 | 0.0 | |
|
JSN Score | |||||||
| Baseline | |||||||
| Mean | 36 | 34 | 31 | 3.0 | 2.9 | 2.9 | |
| Median | 26 | 29 | 24 | 1.0 | 1.0 | 1.0 | |
| Change from baseline | |||||||
| Mean | 2.9 | 1.1a | 0.0a | 0.6 | 0.1a | 0.2 | |
| Median | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
Physical function response
Physical function and disability were assessed using the Health Assessment Questionnaire (HAQ-DI) and the general health-related quality of life questionnaire SF-36.
In Study RA I, all doses/schedules of REMICADE + MTX showed significantly greater improvement from baseline in HAQ-DI and SF-36 physical component summary score averaged over time through week 54 compared to placebo + MTX, and no worsening in the SF-36 mental component summary score. The median (interquartile range) improvement from baseline to week 54 in HAQ-DI was 0.1 (-0.1, 0.5) for the placebo + MTX group and 0.4 (0.1, 0.9) for REMICADE + MTX (p<0.001). Both HAQ-DI and SF-36 effects were maintained through week 102. Approximately 80% of patients in all doses/schedules of REMICADE + MTX remained in the trial through 102 weeks.
In Study RA II, both REMICADE treatment groups showed greater improvement in HAQ-DI from baseline averaged over time through week 54 compared to MTX alone; 0.7 for REMICADE + MTX vs. 0.6 for MTX alone (p≤0.001). No worsening in the SF-36 mental component summary score was observed.
Active Crohn's Disease
The safety and efficacy of single and multiple doses of REMICADE were assessed in two randomized, double-blind, placebo-controlled clinical studies in 653 patients with moderate to severely active Crohn's disease [Crohn's Disease Activity Index (CDAI) ≥220 and ≤400] with an inadequate response to prior conventional therapies. Concomitant stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents were permitted and 92% of patients continued to receive at least one of these medications.
In the single-dose trial9 of 108 patients, 16% (4/25) of placebo patients achieved a clinical response (decrease in CDAI ≥70 points) at week 4 vs. 81% (22/27) of patients receiving 5 mg/kg REMICADE (p<0.001, two-sided, Fisher's Exact test). Additionally, 4% (1/25) of placebo patients and 48% (13/27) of patients receiving 5 mg/kg REMICADE achieved clinical remission (CDAI<150) at week 4.
In a multidose trial (ACCENT I [Study Crohn's I])10, 545 patients received 5 mg/kg at week 0 and were then randomized to one of three treatment groups; the placebo maintenance group received placebo at weeks 2 and 6, and then every 8 weeks; the 5 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then every 8 weeks; and the 10 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then 10 mg/kg every 8 weeks. Patients in response at week 2 were randomized and analyzed separately from those not in response at week 2. Corticosteroid taper was permitted after week 6.
At week 2, 57% (311/545) of patients were in clinical response. At week 30, a significantly greater proportion of these patients in the 5 mg/kg and 10 mg/kg maintenance groups achieved clinical remission compared to patients in the placebo maintenance group (Table 4).
Additionally, a significantly greater proportion of patients in the 5 mg/kg and 10 mg/kg REMICADE maintenance groups were in clinical remission and were able to discontinue corticosteroid use compared to patients in the placebo maintenance group at week 54 (Table 4).
| Single 5 mg/kg Dosea | Three Dose Inductionb | ||||
| Placebo Maintenance | REMICADE Maintenance q 8 wks | ||||
| 5 mg/kg | 10 mg/kg | ||||
|
a REMICADE at week 0 |
|||||
|
b REMICADE 5 mg/kg administered at weeks 0, 2 and 6 |
|||||
|
c p-values represent pairwise comparisons to placebo |
|||||
|
d Of those receiving corticosteroids at baseline |
|||||
| Week 30 Clinical remission | 25/102 25% | 41/104 39% |
48/105 46% | ||
| p-valuec | 0.022 | 0.001 | |||
|
Week 54 Patients in remission able to discontinue corticosteroid used | 6/54 11% | 14/56 25% | 18/53 34% | ||
| p-valuec | 0.059 | 0.005 | |||
Patients in the REMICADE maintenance groups (5 mg/kg and 10 mg/kg) had a longer time to loss of response than patients in the placebo maintenance group ( Figure 1). At weeks 30 and 54, significant improvement from baseline was seen among the 5 mg/kg and 10 mg/kg REMICADE-treated groups compared to the placebo group in the disease specific inflammatory bowel disease questionnaire (IBDQ), particularly the bowel and systemic components, and in the physical component summary score of the general health-related quality of life questionnaire SF-36.
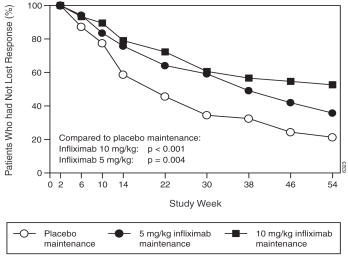
Figure 1 Kaplan-Meier estimate of the proportion of patients who had not lost response through week 54
In a subset of 78 patients who had mucosal ulceration at baseline and who participated in an endoscopic substudy, 13 of 43 patients in the REMICADE maintenance group had endoscopic evidence of mucosal healing compared to 1 of 28 patients in the placebo group at week 10. Of the REMICADE-treated patients showing mucosal healing at week 10, 9 of 12 patients also showed mucosal healing at week 54.
Patients who achieved a response and subsequently lost response were eligible to receive REMICADE on an episodic basis at a dose that was 5 mg/kg higher than the dose to which they were randomized. The majority of such patients responded to the higher dose. Among patients who were not in response at week 2, 59% (92/157) of REMICADE maintenance patients responded by week 14 compared to 51% (39/77) of placebo maintenance patients. Among patients who did not respond by week 14, additional therapy did not result in significantly more responses (see DOSAGE AND ADMINISTRATION).
Fistulizing Crohn's Disease
The safety and efficacy of REMICADE were assessed in 2 randomized, double-blind, placebo-controlled studies in patients with fistulizing Crohn's disease with fistula(s) that were of at least 3 months duration. Concurrent use of stable doses of corticosteroids, 5-aminosalicylates, antibiotics, MTX, 6-mercaptopurine (6-MP) and/or azathioprine (AZA) was permitted.
In the first trial,11 94 patients received three doses of either placebo or REMICADE at weeks 0, 2 and 6. Fistula response (≥50% reduction in number of enterocutaneous fistulas draining upon gentle compression on at least two consecutive visits without an increase in medication or surgery for Crohn's disease) was seen in 68% (21/31) of patients in the 5 mg/kg REMICADE group (p=0.002) and 56% (18/32) of patients in the 10 mg/kg REMICADE group (p=0.021) vs. 26% (8/31) of patients in the placebo arm. The median time to onset of response and median duration of response in REMICADE-treated patients was 2 and 12 weeks, respectively. Closure of all fistula was achieved in 52% of REMICADE-treated patients compared with 13% of placebo-treated patients (p<0.001).
In the second trial (ACCENT II [Study Crohn's II]), patients who were enrolled had to have at least one draining enterocutaneous (perianal, abdominal) fistula. All patients received 5 mg/kg REMICADE at weeks 0, 2 and 6. Patients were randomized to placebo or 5 mg/kg REMICADE maintenance at week 14. Patients received maintenance doses at week 14 and then every eight weeks through week 46. Patients who were in fistula response (fistula response was defined the same as in the first trial) at both weeks 10 and 14 were randomized separately from those not in response. The primary endpoint was time from randomization to loss of response among those patients who were in fistula response.
Among the randomized patients (273 of the 296 initially enrolled), 87% had perianal fistulas and 14% had abdominal fistulas. Eight percent also had rectovaginal fistulas. Greater than 90% of the patients had received previous immunosuppressive and antibiotic therapy.
At week 14, 65% (177/273) of patients were in fistula response. Patients randomized to REMICADE maintenance had a longer time to loss of fistula response compared to the placebo maintenance group ( Figure 2). At week 54, 38% (33/87) of REMICADE-treated patients had no draining fistulas compared with 22% (20/90) of placebo-treated patients (p=0.02). Compared to placebo maintenance, patients on REMICADE maintenance had a trend toward fewer hospitalizations.
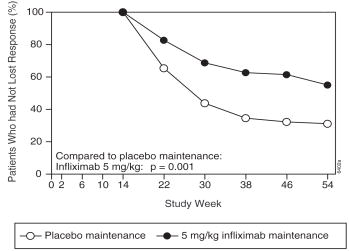
Figure 2 Life table estimates of the proportion of patients who had not lost fistula response through week 54
Patients who achieved a fistula response and subsequently lost response were eligible to receive REMICADE maintenance therapy at a dose that was 5 mg/kg higher than the dose to which they were randomized. Of the placebo maintenance patients, 66% (25/38) responded to 5 mg/kg REMICADE, and 57% (12/21) of REMICADE maintenance patients responded to 10 mg/kg.
Patients who had not achieved a response by week 14 were unlikely to respond to additional doses of REMICADE.
Similar proportions of patients in either group developed new fistulas (17% overall) and similar numbers developed abscesses (15% overall).
Active Crohn's Disease in Pediatric Patients
The safety and efficacy of REMICADE were assessed in a randomized, open-label study (Study Peds Crohn's) in 112 pediatric patients 6 to 17 years old with moderately to severely active Crohn's disease and an inadequate response to conventional therapies. The median age was 13 years and the median Pediatric Crohn's Disease Activity Index (PCDAI) was 40 (on a scale of 0 to 100). All patients were required to be on a stable dose of 6-mercaptopurine, azathioprine, or methotrexate; 35% were also receiving corticosteroids at baseline.
All patients received induction dosing of 5 mg/kg REMICADE at Weeks 0, 2, and 6. At Week 10, 103 patients were randomized to a maintenance regimen of 5 mg/kg REMICADE given either every 8 weeks or every 12 weeks.
At Week 10, 88% of patients were in clinical response (defined as a decrease from baseline in the PCDAI score of 15 points and total PCDAI score of 30 points), and 59% were in clinical remission (defined as PCDAI score of 10 points).
The proportion of pediatric patients achieving clinical response at Week 10 compared favorably with the proportion of adults achieving a clinical response in Study Crohn's I. The study definition of clinical response in Study Peds Crohn's was based on the PCDAI score, whereas the CDAI score was used in the adult Study Crohn's I.
At both Week 30 and Week 54, the proportion of patients in clinical response was greater in the every 8 week treatment group than in the every 12 week treatment group (73% vs. 47% at Week 30, and 64% vs. 33% at Week 54). At both Week 30 and Week 54, the proportion of patients in clinical remission was also greater in the every 8 week treatment group than in the every 12 week treatment group (60% vs. 35% at Week 30, and 56% vs. 24% at Week 54), (Table 5).
For patients in Study Peds Crohn's receiving corticosteroids at baseline, the proportion of patients able to discontinue corticosteroids while in remission at Week 30 was 46% for the every 8 week maintenance group and 33% for the every 12 week maintenance group. At Week 54, the proportion of patients able to discontinue corticosteroids while in remission was 46% for the every 8 week maintenance group and 17% for the every 12 week maintenance group.
|
1Defined as a decrease from baseline in the PCDAI score of ≥ 15 points and total score of ≤ 30 points. |
|||
|
2Defined as a PCDAI score of ≤ 10 points. |
|||
|
* p-value < 0.05 |
|||
|
**p-value < 0.01 |
|||
| 5 mg/kg REMICADE | |||
| Every 8 Week | Every 12 Week | ||
| Treatment Group | Treatment Group | ||
| Patients randomized | 52 | 51 | |
| Clinical Response1 | |||
| Week 30 | 73%** | 47% | |
| Week 54 | 64%** | 33% | |
| Clinical Remission2 | |||
| Week 30 | 60%* | 35% | |
| Week 54 | 56%** | 24% | |
Ankylosing Spondylitis
The safety and efficacy of REMICADE were assessed in a randomized, multicenter, double-blind, placebo-controlled study in 279 patients with active ankylosing spondylitis. Patients were between 18 and 74 years of age, and had ankylosing spondylitis as defined by the modified New York criteria for Ankylosing Spondylitis.12 Patients were to have had active disease as evidenced by both a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score >4 (possible range 0-10) and spinal pain >4 (on a Visual Analog Scale [VAS] of 0-10). Patients with complete ankylosis of the spine were excluded from study participation, and the use of Disease Modifying Anti-Rheumatic Drugs (DMARDs) and systemic corticosteroids were prohibited. Doses of REMICADE 5 mg/kg or placebo were administered intravenously at Weeks 0, 2, 6, 12 and 18.
At 24 weeks, improvement in the signs and symptoms of ankylosing spondylitis, as measured by the proportion of patients achieving a 20% improvement in ASAS response criteria (ASAS 20), was seen in 60% of patients in the REMICADE-treated group vs. 18% of patients in the placebo group (p<0.001). Improvement was observed at week 2 and maintained through week 24 ( Figure 3 and Table 6).
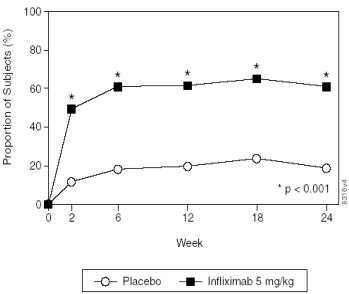
Figure 3 Proportion of patients achieving ASAS 20 response
At 24 weeks, the proportions of patients achieving a 50% and a 70% improvement in the signs and symptoms of ankylosing spondylitis, as measured by ASAS response criteria (ASAS 50 and ASAS 70, respectively), were 44% and 28%, respectively, for patients receiving REMICADE, compared to 9% and 4%, respectively, for patients receiving placebo (p<0.001, REMICADE vs. placebo). A low level of disease activity (defined as a value <20 [on a scale of 0-100 mm] in each of the four ASAS response parameters) was achieved in 22% of REMICADE-treated patients vs. 1% in placebo-treated patients (p<0.001).
| Placebo
(n=78) | REMICADE 5mg/kg
(n=201) | ||||
|
Baseline | 24 Weeks |
Baseline | 24 Weeks |
p-value |
|
|
a measured on a VAS with 0=”none” and 10=”severe” |
|||||
|
b Bath Ankylosing Spondylitis Functional Index (BASFI), average of 10 questions |
|||||
|
c Inflammation, average of last 2 questions on the 6 question BASDAI |
|||||
|
d CRP normal range 0-1.0 mg/dL |
|||||
|
e Spinal mobility normal values: modified Schober's test: >4 cm; chest expansion:>6 cm; tragus to wall: <15 cm; lateral spinal flexion: >10 cm |
|||||
| ASAS 20 response Criteria (Mean) | |||||
| Patient global assessmenta | 6.6 | 6.0 | 6.8 | 3.8 | <0.001 |
| Spinal paina | 7.3 | 6.5 | 7.6 | 4.0 | <0.001 |
| BASFIb | 5.8 | 5.6 | 5.7 | 3.6 | <0.001 |
| Inflammationc | 6.9 | 5.8 | 6.9 | 3.4 | <0.001 |
| Acute Phase Reactants | |||||
| Median CRPd (mg/dL) | 1.7 | 1.5 | 1.5 | 0.4 | <0.001 |
| Spinal Mobility (cm, Mean) | |||||
| Modified Schober's teste | 4.0 | 5.0 | 4.3 | 4.4 | 0.75 |
| Chest expansione | 3.6 | 3.7 | 3.3 | 3.9 | 0.04 |
| Tragus to walle | 17.3 | 17.4 | 16.9 | 15.7 | 0.02 |
| Lateral spinal flexione | 10.6 | 11.0 | 11.4 | 12.9 | 0.03 |
The median improvement from baseline in the general health-related quality of life questionnaire SF-36 physical component summary score at week 24 was 10.2 for the REMICADE group vs. 0.8 for the placebo group (p<0.001). There was no change in the SF-36 mental component summary score in either the REMICADE group or the placebo group.
Results of this study were similar to those seen in a multicenter double-blind, placebo-controlled study of 70 patients with ankylosing spondylitis.
Psoriatic Arthritis
Safety and efficacy of REMICADE were assessed in a multicenter, double-blind, placebo-controlled study in 200 adult patients with active psoriatic arthritis despite DMARD or NSAID therapy (≥ 5 swollen joints and ≥ 5 tender joints) with one or more of the following subtypes: arthritis involving DIP joints (n=49), arthritis mutilans (n=3), asymmetric peripheral arthritis (n=40), polyarticular arthritis (n=100), and spondylitis with peripheral arthritis (n=8). Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Forty-six percent of patients continued on stable doses of methotrexate (≤ 25 mg/week). During the 24-week double-blind phase, patients received either 5 mg/kg REMICADE or placebo at weeks 0, 2, 6, 14, and 22 (100 patients in each group). At week 16, placebo patients with < 10% improvement from baseline in both swollen and tender joint counts were switched to REMICADE induction (early escape). At week 24, all placebo-treated patients crossed over to REMICADE induction. Dosing continued for all patients through week 46.
Clinical response
Treatment with REMICADE resulted in improvement in signs and symptoms, as assessed by the ACR criteria, with 58% of REMICADE-treated patients achieving ACR 20 at week 14, compared with 11% of placebo-treated patients (p < 0.001). The response was similar regardless of concomitant use of methotrexate. Improvement was observed as early as week 2. At 6 months, the ACR 20/50/70 responses were achieved by 54%, 41%, and 27%, respectively, of patients receiving REMICADE compared to 16%, 4%, and 2%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of psoriatic arthritis, although few patients were enrolled with the arthritis mutilans and spondylitis with peripheral arthritis subtypes.
Compared to placebo, treatment with REMICADE resulted in improvements in the components of the ACR response criteria, as well as in dactylitis and enthesopathy (Table 7). The clinical response was maintained through week 54. Similar ACR responses were observed in an earlier randomized, placebo-controlled study of 104 psoriatic arthritis patients, and the responses were maintained through 98 weeks in an open label extension phase.
|
a p<0.001 for percent change from baseline in all components of ACR 20 at week 24, p<0.05 for % of patients with dactylitis, and p=0.004 for % of patients with enthesopathy at week 24 |
||||
|
b Scale 0-68 |
||||
|
c Scale 0-66 |
||||
|
d Visual Analog Scale (0=best, 10=worst) |
||||
|
e Health Assessment Questionnaire, measurement of 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (0=best, 3=worst) |
||||
|
f Normal range 0-0.6 mg/dL |
||||
| Placebo | REMICADE 5mg/kga | |||
| Patients Randomized | (n=100) | (n=100) | ||
| Baseline | Week 24 | Baseline | Week 24 | |
| Parameter (medians) | ||||
| No of Tender Jointsb | 24 | 20 | 20 | 6 |
| No. of Swollen Jointsc | 12 | 9 | 12 | 3 |
| Paind | 6.4 | 5.6 | 5.9 | 2.6 |
| Physician's Global Assessmentd | 6.0 | 4.5 | 5.6 | 1.5 |
| Patient's Global Assessmentd | 6.1 | 5.0 | 5.9 | 2.5 |
| Disability Index (HAQ-DI)e | 1.1 | 1.1 | 1.1 | 0.5 |
| CRP (mg/dL) f | 1.2 | 0.9 | 1.0 | 0.4 |
| % Patients with 1 or more digits with dactylitis | 41 | 33 | 40 | 15 |
| % Patients with enthesopathy | 35 | 36 | 42 | 22 |
Improvement in Psoriasis Area and Severity Index (PASI) in psoriatic arthritis patients with baseline body surface area (BSA) ≥ 3% (n=87 placebo, n=83 REMICADE) was achieved at week 14, regardless of concomitant methotrexate use, with 64% of REMICADE-treated patients achieving at least 75% improvement from baseline vs. 2% of placebo-treated patients; improvement was observed in some patients as early as week 2. At 6 months, the PASI 75 and PASI 90 responses were achieved by 60% and 39%, respectively, of patients receiving REMICADE compared to 1% and 0%, respectively, of patients receiving placebo. The PASI response was generally maintained through week 54. See also CLINICAL STUDIES: Plaque Psoriasis section below.
Radiographic response
Structural damage in both hands and feet was assessed radiographically by the change from baseline in the van der Heijde-Sharp (vdH-S) score, modified by the addition of hand DIP joints. The total modified vdH-S score is a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing (JSN) in the hands and feet. At Week 24, REMICADE-treated patients had less radiographic progression than placebo-treated patients (mean change of -0.70 vs. 0.82, p<0.001). REMICADE-treated patients also had less progression in their erosion scores (-0.56 vs. 0.51 and JSN scores (-0.14 vs. 0.31). The patients in the REMICADE group demonstrated continued inhibition of structural damage at week 54. Most patients showed little or no change in the vdH-S score during this 12-month study (median change of 0 in both patients who initially received REMICADE or placebo). More patients in the placebo group (12%) had readily apparent radiographic progression compared with the REMICADE group (3%).
Physical function
Physical function status was assessed using the HAQ Disability Index (HAQ-DI) and the SF-36 Health Survey. REMICADE-treated patients demonstrated significant improvement in physical function as assessed by HAQ-DI (median percent improvement in HAQ-DI score from baseline to week 14 and 24 of 43% for REMICADE-treated patients vs. 0% for placebo-treated patients).
During the placebo-controlled portion of the trial (24 weeks), 54% of REMICADE-treated patients achieved a clinically meaningful improvement in HAQ-DI (≥ 0.3 unit decrease) compared to 22% of placebo-treated patients. REMICADE-treated patients also demonstrated greater improvement in the SF-36 physical and mental component summary scores than placebo-treated patients. The responses were maintained for up to 2 years in an open label extension study.
Plaque Psoriasis
The safety and efficacy of REMICADE were assessed in three randomized, double-blind, placebo-controlled studies in patients 18 years of age and older with chronic, stable plaque psoriasis involving ≥ 10% BSA, a minimum PASI score of 12, and who were candidates for systemic therapy or phototherapy. Patients with guttate, pustular, or erythrodermic psoriasis were excluded from these studies. No concomitant anti-psoriatic therapies were allowed during the study, with the exception of low-potency topical corticosteroids on the face and groin after week 10 of study initiation.
Study I (EXPRESS) evaluated 378 patients who received placebo or REMICADE at a dose of 5 mg/kg at weeks 0, 2, and 6 (induction therapy), followed by maintenance therapy every 8 weeks. At week 24, the placebo group crossed over to REMICADE induction therapy (5 mg/kg), followed by maintenance therapy every 8 weeks. Patients originally randomized to REMICADE continued to receive REMICADE 5 mg/kg every 8 weeks through week 46. Across all treatment groups, the median baseline PASI score was 21 and the baseline Static Physician Global Assessment (sPGA) score ranged from moderate (52% of patients) to marked (36%) to severe (2%). In addition, 75% of patients had a BSA >20%. Seventy-one percent of patients previously received systemic therapy and 82% received phototherapy.
Study II (EXPRESS II) evaluated 835 patients who received placebo or REMICADE at doses of 3 mg/kg or 5 mg/kg at Weeks 0, 2, and 6 (induction therapy). At week 14, within each REMICADE dose group, patients were randomized to either scheduled (every 8 weeks) or as needed (PRN) maintenance treatment through week 46. At week 16, the placebo group crossed over to REMICADE induction therapy (5 mg/kg), followed by maintenance therapy every 8 weeks. Across all treatment groups, the median baseline PASI score was 18 and 63% of patients had a BSA >20%. Fifty-five percent of patients previously received systemic therapy and 64% received a phototherapy.
Study III (SPIRIT) evaluated 249 patients who had previously received either psoralen plus ultraviolet A treatment (PUVA) or other systemic therapy for their psoriasis. These patients were randomized to receive either placebo or REMICADE at doses of 3 mg/kg or 5 mg/kg at weeks 0, 2, and 6. At week 26, patients with a sPGA score of moderate or worse (greater than or equal to 3 on a scale of 0 to 5) received an additional dose of the randomized treatment. Across all treatment groups, the median baseline PASI score was 19 and the baseline sPGA score ranged from moderate (62% of patients) to marked (22%) to severe (3%). In addition, 75% of patients had a BSA >20%. Of the enrolled patients 114 (46%) received the week 26 additional dose.
In Studies I, II and III, the primary endpoint was the proportion of patients who achieved a reduction in score of at least 75% from baseline at week 10 by the PASI (PASI 75). In Study I and Study III, another evaluated outcome included the proportion of patients who achieved a score of "cleared" or "minimal" by the sPGA. The sPGA is a 6 category scale ranging from "5 = severe" to "0 = cleared" indicating the physician's overall assessment of the psoriasis severity focusing on induration, erythema, and scaling. Treatment success, defined as "cleared" or "minimal", consisted of none or minimal elevation in plaque, up to faint red coloration in erythema, and none or minimal fine scale over < 5% of the plaque.
Study II also evaluated the proportion of patients who achieved a score of “clear” or “excellent” by the relative Physician's Global Assessment (rPGA). The rPGA is a 6 category scale ranging from “6 = worse” to “1 = clear” that was assessed relative to baseline. Overall lesions were graded with consideration to the percent of body involvement as well as overall induration, scaling, and erythema. Treatment success, defined as "clear" or "excellent", consisted of some residual pinkness or pigmentation to marked improvement (nearly normal skin texture; some erythema may be present). The results of these studies are presented in Table 8.
|
* p<0.001 compared with placebo |
|||
|
a Patients with missing data at week 10 were considered as nonresponders. |
|||
|
b Patients with missing data at week 10 were imputed by last observation. |
|||
| Placebo | REMICADE | ||
| 3 mg/kg | 5 mg/kg | ||
| Psoriasis Study I - patients randomizeda
PASI 75 | 77 2 (3%) | --- --- | 301 242 (80%)* |
| sPGA | 3 (4%) | --- | 242 (80%)* |
| Psoriasis Study II - patients randomizeda
PASI 75 | 208 4 (2%) | 313 220 (70%)* | 314 237 (75%)* |
| rPGA | 2 (1%) | 217 (69%)* | 234 (75%)* |
| Psoriasis Study III - patients randomizedb
PASI 75 | 51 3 (6%) | 99 71 (72%)* | 99 87 (88%)* |
| sPGA | 5 (10%) | 71 (72%)* | 89 (90%)* |
In Study I, in the subgroup of patients with more extensive psoriasis who had previously received phototherapy, 85% of patients on 5 mg/kg REMICADE achieved a PASI 75 at week 10 compared with 4% of patients on placebo.
In Study II, in the subgroup of patients with more extensive psoriasis who had previously received phototherapy, 72% and 77% of patients on 3 mg/kg and 5 mg/kg REMICADE achieved a PASI 75 at week 10 respectively compared with 1% on placebo. In Study II, among patients with more extensive psoriasis who had failed or were intolerant to phototherapy, 70% and 78% of patients on 3 mg/kg and 5 mg/kg REMICADE achieved a PASI 75 at week 10 respectively, compared with 2% on placebo.
Maintenance of response was studied in a subset of 292 and 297 REMICADE treated patients in the 3 mg/kg and 5 mg/kg groups; respectively, in Study II. Stratified by PASI response at week 10 and investigational site, patients in the active treatment groups were re-randomized to either a scheduled or as needed maintenance (PRN) therapy, beginning on week 14.
The groups that received a maintenance dose every 8 weeks appear to have a greater percentage of patients maintaining a PASI 75 through week 50 as compared to patients who received the as needed or PRN doses and the best response was maintained with the 5 mg/kg every 8 week dose. These results are shown in Figure 4. At week 46, when REMICADE serum concentrations were at trough level, in the every 8 week dose group, 54% of patients in the 5 mg/kg group compared to 36% in the 3 mg/kg group achieved PASI 75. The lower percentage of PASI 75 responders in the 3mg/kg every 8 week dose group compared to the 5mg/kg group was associated with a lower percentage of patients with detectable trough serum infliximab levels. This may be related in part to higher antibody rates (see ADVERSE REACTIONS: Immunogenicity). In addition, in a subset of patients who had achieved a response at week 10, maintenance of response appears to be greater in patients who received REMICADE every 8 weeks at the 5 mg/kg dose. Regardless of whether the maintenance doses are PRN or every 8 weeks, there is a decline in response in a subpopulation of patients in each group over time. The results of Study I through Week 50 in the 5mg/kg every 8 weeks maintenance dose group were similar to the results from Study II.

Figure 4 Proportion of patients achieving ≥ 75% improvement in PASI from baseline through Week 50; patients randomized at Week 14
Efficacy and safety of REMICADE treatment beyond 50 weeks have not been evaluated in patients with plaque psoriasis.
Ulcerative Colitis
The safety and efficacy of REMICADE were assessed in two randomized, double-blind, placebo-controlled clinical studies in 728 patients with moderately to severely active ulcerative colitis (UC) (Mayo score13 6 to 12 [of possible range 0-12], Endoscopy subscore ≥ 2) with an inadequate response toconventional oral therapies (Studies UC I and UC II). Concomitant treatment with stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents was permitted. Corticosteroi taper was permitted after week 8. Patients were randomized at week 0 to receive either placebo, 5 mg/kg REMICADE or 10 mg/kg REMICADE at weeks 0, 2, 6, and every 8 weeks thereafter through week 46 in Study UC I, and at weeks 0, 2, 6, and every 8 weeks thereafter through week 22 in Study UC II. In Study UC II, patients were allowed to continue blinded therapy to week 46 at the investigator's discretion.
Patients in Study UC I had failed to respond or were intolerant to oral corticosteroids, 6-mercaptopurine (6-MP), or azathioprine (AZA). Patients in Study UC II had failed to respond or were intolerant to the above treatments and/or aminosalicylates. Similar proportions of patients in Studies UC I and UC II were receiving corticosteroids (61% and 51%, respectively), 6-MP/azathioprine (49% and 43%) and aminosalicylates (70% and 75%) at baseline. More patients in Study UC II than UC I were taking solely aminosalicylates for UC (26% vs. 11%, respectively). Clinical response was defined as a decrease from baseline in the Mayo score by ≥ 30% and ≥ 3 points, accompanied by a decrease in the rectal bleeding subscore of ≥ 1 or a rectal bleeding subscore of 0 or 1.
Clinical Response, Clinical Remission, and Mucosal Healing
In both Study UC I and Study UC II, greater percentages of patients in both REMICADE groups achieved clinical response, clinical remission and mucosal healing than in the placebo group. Each of these effects was maintained through the end of each trial (week 54 in Study UC I, and week 30 in Study UC II). In addition, a greater proportion of patients in REMICADE groups demonstrated sustained response and sustained remission than in the placebo groups (Table 9).
Of patients on corticosteroids at baseline, greater proportions of patients in the REMICADE treatment groups were in clinical remission and able to discontinue corticosteroids at week 30 compared with the patients in the placebo treatment groups (22% in REMICADE treatment groups vs. 10% in placebo group in Study UC I; 23% in REMICADE treatment groups vs. 3% in placebo group in Study UC II). In Study UC I, this effect was maintained through week 54 (21% in REMICADE treatment groups vs. 9% in placebo group). The REMICADE-associated response was generally similar in the 5 mg/kg and 10 mg/kg dose groups.
|
* P < 0.001, ** P < 0.01 |
||||||
|
1 Defined as a decrease from baseline in the Mayo score by ≥ 30% and ≥ 3 points, accompanied by a decrease in the rectal bleeding subscore of ≥ 1 or a rectal bleeding subscore of 0 or 1. (The Mayo score consists of the sum of four subscores: stool frequency, rectal bleeding, physician's global assessment and endoscopy findings.) |
||||||
|
2 Defined as a Mayo score ≤2 points, no individual subscore >1. |
||||||
|
3 Defined as a 0 or 1 on the endoscopy subscore of the Mayo score. |
||||||
|
4 Patients who had a prohibited change in medication, had an ostomy or colectomy, or discontinued study infusions |
||||||
|
due to lack of efficacy are considered to not be in clinical response, clinical remission or mucosal healing from the time of the event onward. |
||||||
| Study UC I | Study UC II | |||||
| Placebo | 5 mg/kg REMICADE | 10 mg/kg REMICADE | Placebo | 5 mg/kg REMICADE | 10 mg/kg REMICADE | |
| Patients randomized | 121 | 121 | 122 | 123 | 121 | 120 |
| Clinical Response1, 4 | ||||||
| Week 8 | 37% | 69%* | 62%* | 29% | 65%* | 69%* |
| Week 30 | 30% | 52%* | 51%** | 26% | 47%* | 60%* |
| Week 54 | 20% | 45%* | 44%* | NA | NA | NA |
| Sustained Response4 | ||||||
| (Clinical response at both Week 8 and 30) | 23% | 49%* | 46%* | 15% | 41%* | 53%* |
| (Clinical response at Weeks 8, 30, and 54) | 14% | 39%* | 37%* | NA | NA | NA |
| Clinical Remission2, 4 | ||||||
| Week 8 | 15% | 39%* | 32%** | 6% | 34%* | 28%* |
| Week 30 | 16% | 34%** | 37%* | 11% | 26%** | 36%* |
| Week 54 | 17% | 35%** | 34%** | NA | NA | NA |
| Sustained Remission4 | ||||||
| (Clinical remission at both Week 8 and 30) | 8% | 23%** | 26%* | 2% | 15%* | 23%* |
| (Clinical remission at Weeks 8, 30 and 54) | 7% | 20%** | 20%** | NA | NA | NA |
| Mucosal Healing3, 4 | ||||||
| Week 8 | 34% | 62%* | 59%* | 31% | 60%* | 62%* |
| Week 30 | 25% | 50%* | 49%* | 30% | 46%** | 57%* |
| Week 54 | 18% | 45%* | 47%* | NA | NA | NA |
The improvement with REMICADE was consistent across all Mayo subscores through week 54 (Study UC I shown in Table 10; Study UC II through week 30 was similar).
| Study UC I | |||
| REMICADE | |||
| Placebo | 5 mg/kg | 10 mg/kg | |
| (n=121) | (n=121) | (n=122) | |
| Stool frequency | |||
| Baseline | 17% | 17% | 10% |
| Week 8 | 35% | 60% | 58% |
| Week 30 | 35% | 51% | 53% |
| Week 54 | 31% | 52% | 51% |
| Rectal bleeding | |||
| Baseline | 54% | 40% | 48% |
| Week 8 | 74% | 86% | 80% |
| Week 30 | 65% | 74% | 71% |
| Week 54 | 62% | 69% | 67% |
| Physician's global assessment | |||
| Baseline | 4% | 6% | 3% |
| Week 8 | 44% | 74% | 64% |
| Week 30 | 36% | 57% | 55% |
| Week 54 | 26% | 53% | 53% |
| Endoscopy findings | |||
| Baseline | 0% | 0% | 0% |
| Week 8 | 34% | 62% | 59% |
| Week 30 | 26% | 51% | 52% |
| Week 54 | 21% | 50% | 51% |
INDICATIONS AND USAGE
Rheumatoid Arthritis
REMICADE, in combination with methotrexate, is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis.
Crohn's Disease
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in adult and pediatric patients with moderately to severely active Crohn's disease who have had an inadequate response to conventional therapy(see Boxed WARNINGS, WARNINGS, and PRECAUTIONS-Pediatric Use).
REMICADE is indicated for reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in adult patients with fistulizing Crohn's disease.
Ankylosing Spondylitis
REMICADE is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis.
Psoriatic Arthritis
REMICADE is indicated for reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function in patients with psoriatic arthritis.
Plaque Psoriasis
REMICADE is indicated for the treatment of adult patients with chronic severe (i.e., extensive and /or disabling) plaque psoriasis who are candidates for systemic therapy and when other systemic therapies are medically less appropriate. REMICADE should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician (See Boxed WARNINGS, WARNINGS, and PRECAUTIONS).
Ulcerative Colitis
REMICADE is indicated for reducing signs and symptoms, inducing and maintaining clinical remission and mucosal healing, and eliminating corticosteroid use in patients with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy.
CONTRAINDICATIONS
REMICADE at doses >5 mg/kg should not be administered to patients with moderate to severe heart failure. In a randomized study evaluating REMICADE in patients with moderate to severe heart failure (New York Heart Association [NYHA] Functional Class III/IV), REMICADE treatment at 10 mg/kg was associated with an increased incidence of death and hospitalization due to worsening heart failure (see WARNINGS and ADVERSE REACTIONS, Patients with Heart Failure).
REMICADE should not be re-administered to patients who have experienced a severe hypersensitivity reaction to REMICADE. Additionally, REMICADE should not be administered to patients with known hypersensitivity to inactive components of the product or to any murine proteins.
WARNINGS
RISK OF INFECTIONS
(See Boxed WARNINGS)
Serious infections, including sepsis and pneumonia, have been reported in patients receiving TNF-blocking agents. Some of these infections have been fatal. Although some of the serious infections in patients treated with REMICADE have occurred in patients on concomitant immunosuppressive therapy which in addition to their underlying disease, could further predispose them to infections, some patients who were hospitalized or had a fatal outcome from infection were treated with REMICADE alone.
REMICADE should not be given to patients with a clinically important, active infection. Caution should be exercised when considering the use of REMICADE in patients with a chronic infection or a history of recurrent infection. Patients should be monitored for signs and symptoms of infection while on or after treatment with REMICADE. New infections should be closely monitored. If a patient develops a serious infection, REMICADE therapy should be discontinued (see ADVERSE REACTIONS: Infections).
Cases of tuberculosis, histoplasmosis, coccidioidomycosis, listeriosis, pneumocystosis, other bacterial, mycobacterial and fungal infections have been observed in patients receiving REMICADE. Patients should be evaluated for tuberculosis risk factors and be tested for latent tuberculosis infection. Treatment of latent tuberculosis infections should be initiated prior to therapy with REMICADE. When tuberculin skin testing is performed for latent tuberculosis infection an induration size of 5 mm or greater should be considered positive, even if vaccinated previously with Bacille Calmette-Guerin (BCG).
Patients receiving REMICADE should be monitored closely for signs and symptoms of active tuberculosis, particularly since tests for latent tuberculosis infection may be falsely negative. The possibility of undetected latent tuberculosis should be considered, especially in patients who have immigrated from or traveled to countries with a high prevalence of tuberculosis or had close contact with a person with active tuberculosis. All patients treated with REMICADE should have a thorough history taken prior to initiating therapy. Some patients who have previously received treatment for latent or active tuberculosis have developed active tuberculosis while being treated with REMICADE. Anti-tuberculosis therapy should be considered prior to initiation of REMICADE in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed. Anti-tuberculosis therapy prior to initiating REMICADE should also be considered in patients who have several or highly significant risk factors for tuberculosis infection14 and have a negative test for latent tuberculosis. The decision to initiate anti-tuberculosis therapy in these patients should only be made following consultation with a physician with expertise in the treatment of tuberculosis and taking into account both the risk for latent tuberculosis infection and the risks of anti-tuberculosis therapy.
For patients who have resided in regions where histoplasmosis or coccidioidomycosis is endemic, the benefits and risks of REMICADE treatment should be carefully considered before initiation of REMICADE therapy.
Serious infections were seen in clinical studies with concurrent use of anakinra and another TNFα-blocking agent, etanercept, with no added clinical benefit compared to etanercept alone. Because of the nature of the adverse events seen with combination of etanercept and anakinra therapy, similar toxicities may also result from the combination of anakinra and other TNFα-blocking agents. Therefore, the combination of REMICADE and anakinra is not recommended.
HEPATOSPLENIC T-CELL LYMPHOMAS
(See Boxed WARNINGS)
Rare postmarketing cases of hepatosplenic T-cell lymphomas have been reported in adolescent and young adult patients with Crohn's disease treated with REMICADE. All of these reports have occurred in patients on concomitant treatment with azathioprine or 6-mercaptopurine. The clinical course of this disease is very aggressive with a fatal outcome in most patients within 2 years of diagnosis.15 The causal relationship of hepatosplenic T-cell lymphoma to REMICADE therapy remains unclear.
Hepatitis B Virus Reactivation
Use of TNF blockers, including REMICADE has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation. Patients at risk for HBV infection should be evaluated for prior evidence of HBV infection before initiating TNF blocker therapy. Prescribers should exercise caution in prescribing TNF blockers, including REMICADE, for patients identified as carriers of HBV. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with TNF blockers should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, TNF blockers should be stopped and antiviral therapy with appropriate supportive treatment should be initiated. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, prescribers should exercise caution when considering resumption of TNF blocker therapy in this situation and monitor patients closely.
Hepatotoxicity
Severe hepatic reactions, including acute liver failure, jaundice, hepatitis and cholestasis have been reported rarely in postmarketing data in patients receiving REMICADE. Autoimmune hepatitis has been diagnosed in some of these cases. Severe hepatic reactions occurred between two weeks to more than a year after initiation of REMICADE; elevations in hepatic aminotransferase levels were not noted prior to discovery of the liver injury in many of these cases. Some of these cases were fatal or necessitated liver transplantation. Patients with symptoms or signs of liver dysfunction should be evaluated for evidence of liver injury. If jaundice and/or marked liver enzyme elevations (e.g., ≥5 times the upper limit of normal) develops, REMICADE should be discontinued, and a thorough investigation of the abnormality should be undertaken. In clinical trials, mild or moderate elevations of ALT and AST have been observed in patients receiving REMICADE without progression to severe hepatic injury (see ADVERSE REACTIONS, Hepatotoxicity).
Patients with Heart Failure
REMICADE has been associated with adverse outcomes in patients with heart failure, and should be used in patients with heart failure only after consideration of other treatment options. The results of a randomized study evaluating the use of REMICADE in patients with heart failure (NYHA Functional Class III/IV) suggested higher mortality in patients who received 10 mg/kg REMICADE, and higher rates of cardiovascular adverse events at doses of 5 mg/kg and 10 mg/kg. There have been post-marketing reports of worsening heart failure, with and without identifiable precipitating factors, in patients taking REMICADE. There have also been rare post-marketing reports of new onset heart failure, including heart failure in patients without known pre-existing cardiovascular disease. Some of these patients have been under 50 years of age. If a decision is made to administer REMICADE to patients with heart failure, they should be closely monitored during therapy, and REMICADE should be discontinued if new or worsening symptoms of heart failure appear. (See CONTRAINDICATIONS and ADVERSE REACTIONS, Patients with Heart Failure.)
Hematologic Events
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia, some with a fatal outcome, have been reported in patients receiving REMICADE. The causal relationship to REMICADE therapy remains unclear. Although no high-risk group(s) has been identified, caution should be exercised in patients being treated with REMICADE who have ongoing or a history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) while on REMICADE. Discontinuation of REMICADE therapy should be considered in patients who develop significant hematologic abnormalities.
Hypersensitivity
REMICADE has been associated with hypersensitivity reactions that vary in their time of onset and required hospitalization in some cases. Most hypersensitivity reactions, which include urticaria, dyspnea, and/or hypotension, have occurred during or within 2 hours of REMICADE infusion.
However, in some cases, serum sickness-like reactions have been observed in patients after initial REMICADE therapy (i.e., as early as after the second dose), and when REMICADE therapy was reinstituted following an extended period without REMICADE treatment. Symptoms associated with these reactions include fever, rash, headache, sore throat, myalgias, polyarthralgias, hand and facial edema and/or dysphagia. These reactions were associated with marked increase in antibodies to infliximab, loss of detectable serum concentrations of infliximab, and possible loss of drug efficacy.
REMICADE should be discontinued for severe hypersensitivity reactions (see also CONTRAINDICATIONS). Medications for the treatment of hypersensitivity reactions (e.g., acetaminophen, antihistamines, corticosteroids and/or epinephrine) should be available for immediate use in the event of a reaction (see ADVERSE REACTIONS: Infusion-related Reactions).
Neurologic Events
REMICADE and other agents that inhibit TNF have been associated in rare cases with optic neuritis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis, and CNS manifestation of systemic vasculitis. Prescribers should exercise caution in considering the use of REMICADE in patients with pre-existing or recent onset of central nervous system demyelinating or seizure disorders. Discontinuation of REMICADE should be considered in patients who develop significant central nervous system adverse reactions.
Malignancies
In the controlled portions of clinical trials of some TNF-blocking agents including REMICADE, more malignancies (excluding lymphoma and nonmelanoma skin cancer [NMSC]) have been observed in patients receiving those TNF-blockers compared with control patients. During the controlled portions of REMICADE trials in patients with moderately to severely active rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 14 patients were diagnosed with malignancies (excluding lymphoma and NMSC) among 4019 REMICADE-treated patients vs. 1 among 1597 control patients (at a rate of 0.52/100 patient-years among REMICADE-treated patients vs. a rate of 0.11/100 patient-years among control patients), with median duration of follow-up 0.5 years for REMICADE-treated patients and 0.4 years for control patients. Of these, the most common malignancies were breast, colorectal, and melanoma. The rate of malignancies among REMICADE-treated patients was similar to that expected in the general population whereas the rate in control patients was lower than expected.
In the controlled portions of clinical trials of all the TNF-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients. In the controlled and open-label portions of REMICADE clinical trials, 5 patients developed lymphomas among 5707 patients treated with REMICADE (median duration of follow-up 1.0 years) vs. 0 lymphomas in 1600 control patients (median duration of follow-up 0.4 years). In rheumatoid arthritis patients, 2 lymphomas were observed for a rate of 0.08 cases per 100 patient-years of follow-up, which is approximately 3-fold higher than expected in the general population. In the combined clinical trial population for rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 5 lymphomas were observed for a rate of 0.10 cases per 100 patient-years of follow-up, which is approximately 4-fold higher than expected in the general population. Patients with Crohn's disease, rheumatoid arthritis or plaque psoriasis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF-blocking therapy.
In a clinical trial exploring the use of REMICADE in patients with moderate to severe chronic obstructive pulmonary disease (COPD), more malignancies, the majority of lung or head and neck origin, were reported in REMICADE-treated patients compared with control patients. All patients had a history of heavy smoking (see ADVERSE REACTIONS, Malignancies). Prescribers should exercise caution when considering the use of REMICADE in patients with moderate to severe COPD.
Psoriasis patients should be monitored for nonmelanoma skin cancers (NMSCs), particularly those patients who have had prior prolonged phototherapy treatment. In the maintenance portion of clinical trials for REMICADE, NMSCs were more common in patients with previous phototherapy (see ADVERSE REACTIONS: Adverse Reactions in Psoriasis Studies).
The potential role of TNF-blocking therapy in the development of malignancies is not known (see ADVERSE REACTIONS, Malignancies). Rates in clinical trials for REMICADE cannot be compared to rates in clinical trials of other TNF-blockers and may not predict rates observed in a broader patient population. Caution should be exercised in considering REMICADE treatment in patients with a history of malignancy or in continuing treatment in patients who develop malignancy while receiving REMICADE.
PRECAUTIONS
Autoimmunity
Treatment with REMICADE may result in the formation of autoantibodies and, rarely, in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with REMICADE, treatment should be discontinued (see ADVERSE REACTIONS, Autoantibodies/Lupus-like Syndrome).
Vaccinations
No data are available on the response to vaccination with live vaccines or on the secondary transmission of infection by live vaccines in patients receiving anti-TNF therapy. It is recommended that live vaccines not be given concurrently.
It is recommended that all pediatric Crohn's disease patients be brought up to date with all vaccinations prior to initiating REMICADE therapy. The interval between vaccination and initiation of REMICADE therapy should be in accordance with current vaccination guidelines.
Information for Patients
Patients developing signs and symptoms of infection should seek medical evaluation immediately.
Patients or their caregivers should be provided the REMICADE Medication Guide and provided an opportunity to read it and ask questions prior to each treatment infusion session. Because caution should be exercised in administering REMICADE to patients with clinically important active infections, it is important that the patient's overall health be assessed at each treatment visit and any questions resulting from the patient's or caregiver's reading of the Medication Guide be discussed.
Drug Interactions
Concurrent administration of etanercept (another TNFα-blocking agent) and anakinra (an interleukin-1 receptor antagonist) has been associated with an increased risk of serious infections, and increased risk of neutropenia and no additional benefit compared to these medicinal products alone. Other TNFα-blocking agents (including REMICADE) used in combination with anakinra may also result in similar toxicities (see WARNINGS, RISK OF INFECTIONS).
Specific drug interaction studies, including interactions with MTX, have not been conducted. The majority of patients in rheumatoid arthritis or Crohn's disease clinical studies received one or more concomitant medications. In rheumatoid arthritis, concomitant medications besides MTX were nonsteroidal anti-inflammatory agents, folic acid, corticosteroids and/or narcotics. Concomitant Crohn's disease medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates. In psoriatic arthritis clinical trials, concomitant medications included MTX in approximately half of the patients as well as nonsteroidal anti-inflammatory agents, folic acid and corticosteroids.
Patients with Crohn's disease who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants (see ADVERSE REACTIONS, Immunogenicity and Infusion-related Reactions). Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of Crohn's disease including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
Carcinogenesis, Mutagenesis and Impairment of Fertility
A repeat dose toxicity study was conducted with mice given cV1q anti-mouse TNFα to evaluate tumorigenicity. CV1q is an analogous antibody that inhibits the function of TNFα in mice. Animals were assigned to 1 of 3 dose groups: control, 10 mg/kg or 40 mg/kg cV1q given weekly for 6 months. The weekly doses of 10 mg/kg and 40 mg/kg are 2 and 8 times, respectively, the human dose of 5 mg/kg for Crohn's disease. Results indicated that cV1q did not cause tumorigenicity in mice. No clastogenic or mutagenic effects of infliximab were observed in the in vivo mouse micronucleus test or the Salmonella-Escherichia coli (Ames) assay, respectively. Chromosomal aberrations were not observed in an assay performed using human lymphocytes. The significance of these findings for human risk is unknown. It is not known whether infliximab can impair fertility in humans. No impairment of fertility was observed in a fertility and general reproduction toxicity study with the analogous mouse antibody used in the 6-month chronic toxicity study.
Pregnancy Category B
Since infliximab does not cross-react with TNFα in species other than humans and chimpanzees, animal reproduction studies have not been conducted with REMICADE. No evidence of maternal toxicity, embryotoxicity or teratogenicity was observed in a developmental toxicity study conducted in mice using an analogous antibody that selectively inhibits the functional activity of mouse TNFα. Doses of 10 to 15 mg/kg in pharmacodynamic animal models with the anti-TNF analogous antibody produced maximal pharmacologic effectiveness. Doses up to 40 mg/kg were shown to produce no adverse effects in animal reproduction studies. It is not known whether REMICADE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. REMICADE should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether REMICADE is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for adverse reactions in nursing infants from REMICADE, women should not breast-feed their infants while taking REMICADE. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients with moderately to severely active Crohn's disease who have had an inadequate response to conventional therapy (see Boxed WARNINGS, WARNINGS, INDICATIONS AND USAGE, PRECAUTIONS-Vaccinations, DOSAGE AND ADMINISTRATION, CLINICAL STUDIES-Active Crohn's Disease in Pediatric Patients and ADVERSE REACTIONS - Adverse Reactions in Pediatric Crohn's Disease).
REMICADE has not been studied in children with Crohn's disease < 6 years of age. The longer term (greater than one year) safety and effectiveness of REMICADE in pediatric Crohn's disease patients have not been established in clinical trials.
Safety and effectiveness of REMICADE in pediatric patients with ulcerative colitis and plaque psoriasis have not been established.
The safety and efficacy of REMICADE in patients with juvenile rheumatoid arthritis (JRA) were evaluated in a multicenter, randomized, placebo-controlled, double-blind study for 14 weeks, followed by a double-blind, all-active treatment extension, for a maximum of 44 weeks. Patients with active JRA between the ages of 4 and 17 years who had been treated with MTX for at least 3 months were enrolled. Concurrent use of folic acid, oral corticosteroids (≤0.2 mg/kg/day of prednisone or equivalent), NSAIDs, and/or DMARDS was permitted.
Doses of 3 mg/kg REMICADE or placebo were administered intravenously at weeks 0, 2 and 6. Patients randomized to placebo crossed-over to receive 6 mg/kg REMICADE at weeks 14, 16, and 20, and then every 8 weeks through week 44. Patients who completed the study continued to receive open-label treatment with REMICADE for up to 2 years in a companion extension study.
The study failed to establish the efficacy of REMICADE in the treatment of JRA. Key observations in the study included a high placebo response rate and a higher rate of immunogenicity than what has been observed in adults. Additionally, a higher rate of clearance of infliximab was observed than had been observed in adults (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
A total of 60 patients with JRA were treated with doses of 3 mg/kg and 57 patients were treated with doses of 6 mg/kg. The proportion of patients with infusion reactions who received 3 mg/kg REMICADE was 35% (21/60) over 52 weeks compared with 18% (10/57) in patients who received 6 mg/kg over 38 weeks. The most common infusion reactions reported were vomiting, fever, headache, and hypotension. In the 3 mg/kg REMICADE group, 4 patients had a serious infusion reaction and 3 patients reported a possible anaphylactic reaction (2 of which were among the serious infusion reactions). In the 6 mg/kg REMICADE group, 2 patients had a serious infusion reaction, one of whom had a possible anaphylactic reaction. Two of the 6 patients who experienced serious infusion reactions received REMICADE by rapid infusion (duration of less than 2 hours). Antibodies to infliximab developed in 38% (20/53) of patients who received 3 mg/kg REMICADE compared with 12% (6/49) of patients who received 6 mg/kg.
A total of 68% (41/60) of patients who received 3 mg/kg REMICADE in combination with MTX experienced an infection over 52 weeks compared with 65% (37/57) of patients who received 6 mg/kg REMICADE in combination with MTX over 38 weeks. The most commonly reported infections were upper respiratory tract infection and pharyngitis and the most commonly reported serious infection was pneumonia. Other notable infections included primary varicella infection in 1 patient and herpes zoster in 1 patient.
Geriatric Use
In rheumatoid arthritis and plaque psoriasis clinical trials, no overall differences were observed in effectiveness or safety in 181 patients with rheumatoid arthritis and 75 patients with plaque psoriasis, aged 65 or older who received REMICADE, compared to younger patients although the incidence of serious adverse events in patients aged 65 or older was higher in both REMICADE and control groups compared to younger patients. In Crohn's disease, ulcerative colitis, ankylosing spondylitis and psoriatic arthritis studies, there were insufficient numbers of patients aged 65 and over to determine whether they respond differently from patients aged 18 to 65. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly (see ADVERSE REACTIONS, Infections).
ADVERSE REACTIONS
The data described herein reflect exposure to REMICADE in 4779 adult patients (1304 patients with rheumatoid arthritis, 1106 patients with Crohn's disease, 202 with ankylosing spondylitis, 293 with psoriatic arthritis, 484 with ulcerative colitis, 1373 with plaque psoriasis, and 17 patients with other conditions), including 2625 patients exposed beyond 30 weeks and 374 exposed beyond one year. (For information on adverse reactions in pediatric patients see ADVERSE REACTIONS – Adverse Reactions in Pediatric Crohn's Disease.) One of the most common reasons for discontinuation of treatment was infusion-related reactions (e.g. dyspnea, flushing, headache and rash). Adverse events have been reported in a higher proportion of rheumatoid arthritis patients receiving the 10 mg/kg dose than the 3 mg/kg dose, however, no differences were observed in the frequency of adverse events between the 5 mg/kg dose and 10 mg/kg dose in patients with Crohn's disease.
Infusion-related Reactions
Infusion reactions
An infusion reaction was defined in clinical trials as any adverse event occurring during an infusion or within 1 to 2 hours after an infusion. Approximately 20% of REMICADE-treated patients in all clinical studies experienced an infusion reaction compared to approximately 10% of placebo-treated patients. Among all REMICADE infusions, 3% were accompanied by nonspecific symptoms such as fever or chills, 1% were accompanied by cardiopulmonary reactions (primarily chest pain, hypotension, hypertension or dyspnea), and <1% were accompanied by pruritus, urticaria, or the combined symptoms of pruritus/urticaria and cardiopulmonary reactions. Serious infusion reactions occurred in <1% of patients and included anaphylaxis, convulsions, erythematous rash and hypotension. Approximately 3% of patients discontinued REMICADE because of infusion reactions, and all patients recovered with treatment and/or discontinuation of the infusion. REMICADE infusions beyond the initial infusion were not associated with a higher incidence of reactions. The infusion reaction rates remained stable in psoriasis through 1 year in psoriasis Study I. In psoriasis Study II, the rates were variable over time and somewhat higher following the final infusion than after the initial infusion. Across the 3 psoriasis studies, the percent of total infusions resulting in infusion reactions (i.e. an adverse event occurring within 1 to 2 hours) was 7% in the 3 mg/kg group, 4% in the 5 mg/kg group, and 1% in the placebo group.
Patients who became positive for antibodies to infliximab were more likely (approximately 2- to 3-fold) to have an infusion reaction than were those who were negative. Use of concomitant immunosuppressant agents appeared to reduce the frequency of antibodies to infliximab and infusion reactions (see ADVERSE REACTIONS, Immunogenicity and PRECAUTIONS, Drug Interactions).
In post-marketing experience, cases of anaphylactic-like reactions, including laryngeal/pharyngeal edema and severe bronchospasm, and seizure have been associated with REMICADE administration.
Delayed Reactions/Reactions following readministration
Plaque Psoriasis
In psoriasis studies, approximately 1% of REMICADE-treated patients experienced a possible delayed hypersensitivity reaction, generally reported as serum sickness or a combination of arthralgia and/or myalgia with fever and/or rash. These reactions generally occurred within two weeks after repeat infusion.
Crohn's disease
In a study where 37 of 41 patients with Crohn's disease were retreated with infliximab following a 2 to 4 year period without infliximab treatment, 10 patients experienced adverse events manifesting 3 to 12 days following infusion of which 6 were considered serious. Signs and symptoms included myalgia and/or arthralgia with fever and/or rash, with some patients also experiencing pruritus, facial, hand or lip edema, dysphagia, urticaria, sore throat, and headache. Patients experiencing these adverse events had not experienced infusion-related adverse events associated with their initial infliximab therapy. These adverse events occurred in 39% (9/23) of patients who had received liquid formulation which is no longer in use and 7% (1/14) of patients who received lyophilized formulation. The clinical data are not adequate to determine if occurrence of these reactions is due to differences in formulation. Patients' signs and symptoms improved substantially or resolved with treatment in all cases. There are insufficient data on the incidence of these events after drug-free intervals of 1 to 2 years. These events have been observed only infrequently in clinical studies and post-marketing surveillance with retreatment intervals up to 1 year.
Infections
In REMICADE clinical studies, treated infections were reported in 36% of REMICADE-treated patients (average of 51 weeks of follow-up) and in 25% of placebo-treated patients (average of 37 weeks of follow-up). The infections most frequently reported were respiratory tract infections (including sinusitis, pharyngitis, and bronchitis) and urinary tract infections. Among REMICADE-treated patients, serious infections included pneumonia, cellulitis, abscess, skin ulceration, sepsis, and bacterial infection. In clinical trials, 7 opportunistic infections were reported; 2 cases each of coccidioidomycosis (1 case was fatal) and histoplasmosis (1 case was fatal), and 1 case each of pneumocystosis, nocardiosis and cytomegalovirus. Tuberculosis was reported in 14 patients, 4 of whom died due to miliary tuberculosis. Other cases of tuberculosis, including disseminated tuberculosis, also have been reported post-marketing. Most of these cases of tuberculosis occurred within the first 2 months after initiation of therapy with REMICADE and may reflect recrudescence of latent disease (see WARNINGS, RISK OF INFECTIONS). In the 1 year placebo-controlled studies RA I and RA II, 5.3% of patients receiving REMICADE every 8 weeks with MTX developed serious infections as compared to 3.4% of placebo patients receiving MTX. Of 924 patients receiving REMICADE, 1.7% developed pneumonia and 0.4% developed TB, when compared to 0.3% and 0.0% in the placebo arm respectively. In a shorter (22-week) placebo-controlled study of 1082 RA patients randomized to receive placebo, 3 mg/kg or 10 mg/kg REMICADE infusions at 0, 2, and 6 weeks, followed by every 8 weeks with MTX, serious infections were more frequent in the 10 mg/kg REMICADE group (5.3%) than the 3 mg/kg or placebo groups (1.7% in both). During the 54 weeks Crohn's II Study, 15% of patients with fistulizing Crohn's disease developed a new fistula-related abscess.
In REMICADE clinical studies in patients with ulcerative colitis, infections treated with antimicrobials were reported in 27% of REMICADE-treated patients (average of 41 weeks of follow-up) and in 18% of placebo-treated patients (average 32 weeks of follow-up). The types of infections, including serious infections, reported in patients with ulcerative colitis were similar to those reported in other clinical studies.
In post-marketing experience in the various indications, infections have been observed with various pathogens including viral, bacterial, fungal, and protozoal organisms. Infections have been noted in all organ systems and have been reported in patients receiving REMICADE alone or in combination with immunosuppressive agents.
The onset of serious infections may be preceded by constitutional symptoms such as fever, chills, weight loss, and fatigue. The majority of serious infections, however, may also be preceded by signs or symptoms localized to the site of the infection.
Autoantibodies/Lupus-like Syndrome
Approximately half of REMICADE-treated patients in clinical trials who were antinuclear antibody (ANA) negative at baseline developed a positive ANA during the trial compared with approximately one-fifth of placebo-treated patients. Anti-dsDNA antibodies were newly detected in approximately one-fifth of REMICADE-treated patients compared with 0% of placebo-treated patients. Reports of lupus and lupus-like syndromes, however, remain uncommon.
Malignancies
In controlled trials, more REMICADE-treated patients developed malignancies than placebo-treated patients. (See WARNINGS, Malignancies.)
In a randomized controlled clinical trial exploring the use of REMICADE in patients with moderate to severe COPD who were either current smokers or ex-smokers, 157 patients were treated with REMICADE at doses similar to those used in rheumatoid arthritis and Crohn's disease. Nine of these REMICADE-treated patients developed a malignancy, including 1 lymphoma, for a rate of 7.67 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 3.51 - 14.56). There was one reported malignancy among 77 control patients for a rate of 1.63 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 0.04 - 9.10). The majority of the malignancies developed in the lung or head and neck.
Malignancies, including non-Hodgkin's lymphoma and Hodgkin's disease, have also been reported in patients receiving REMICADE during post-approval use.
Patients with Heart Failure
In a randomized study evaluating REMICADE in moderate to severe heart failure (NYHA Class III/IV; left ventricular ejection fraction ≤35%), 150 patients were randomized to receive treatment with 3 infusions of REMICADE 10 mg/kg, 5 mg/kg, or placebo, at 0, 2, and 6 weeks. Higher incidences of mortality and hospitalization due to worsening heart failure were observed in patients receiving the 10 mg/kg REMICADE dose. At 1 year, 8 patients in the 10 mg/kg REMICADE group had died compared with 4 deaths each in the 5 mg/kg REMICADE and the placebo groups. There were trends towards increased dyspnea, hypotension, angina, and dizziness in both the 10 mg/kg and 5 mg/kg REMICADE treatment groups, versus placebo. REMICADE has not been studied in patients with mild heart failure (NYHA Class I/II). (See CONTRAINDICATIONS and WARNINGS, Patients with Heart Failure.)
Immunogenicity
Treatment with REMICADE can be associated with the development of antibodies to infliximab. The incidence of antibodies to infliximab in patients given a 3-dose induction regimen followed by maintenance dosing was approximately 10% as assessed through 1 to 2 years of REMICADE treatment. A higher incidence of antibodies to infliximab was observed in Crohn's disease patients receiving REMICADE after drug free intervals >16 weeks. In a study of psoriatic arthritis, where 191 patients received 5 mg/kg with or without MTX, antibodies to infliximab occurred in 15% of patients. The majority of antibody-positive patients had low titers. Patients who were antibody-positive were more likely to have higher rates of clearance, reduced efficacy and to experience an infusion reaction (see ADVERSE REACTIONS, Infusion-related Reactions) than were patients who were antibody negative. Antibody development was lower among rheumatoid arthritis and Crohn's disease patients receiving immunosuppressant therapies such as 6-MP/AZA or MTX.
In the psoriasis Study II, which included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 36% of patients treated with 5 mg/kg every 8 weeks for 1 year, and in 51% of patients treated with 3 mg/kg every 8 weeks for 1 year. In the psoriasis Study III, which also included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 20% of patients treated with 5 mg/kg induction (weeks 0, 2 and 6), and in 27% of patients treated with 3 mg/kg induction. Despite the increase in antibody formation, the infusion reaction rates in Studies I and II in patients treated with 5 mg/kg induction followed by every 8 week maintenance for one year and in Study III in patients treated with 5 mg/kg induction (14.1%-23.0%) and serious infusion reaction rates (<1%) were similar to those observed in other study populations. The clinical significance of apparent increased immunogenicity on efficacy and infusion reactions in psoriasis patients as compared to patients with other diseases treated with REMICADE over the long term is not known.
The data reflect the percentage of patients whose test results were positive for antibodies to infliximab in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to infliximab with the incidence of antibodies to other products may be misleading.
Hepatotoxicity
Severe liver injury, including acute liver failure and autoimmune hepatitis, has been reported rarely in patients receiving REMICADE (see WARNINGS, Hepatotoxicity). Reactivation of hepatitis B virus has occurred in patients receiving TNF-blocking agents, including REMICADE, who are chronic carriers of this virus (see WARNINGS, Hepatitis B Virus Reactivation).
In clinical trials in rheumatoid arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, plaque psoriasis, and psoriatic arthritis, elevations of aminotransferases were observed (ALT more common than AST) in a greater proportion of patients receiving REMICADE than in controls (Table 11), both when REMICADE was given as monotherapy and when it was used in combination with other immunosuppressive agents. In general, patients who developed ALT and AST elevations were asymptomatic, and the abnormalities decreased or resolved with either continuation or discontinuation of REMICADE, or modification of concomitant medications.
|
1Placebo patients received methotrexate while REMICADE patients received both REMICADE and methotrexate. Median follow-up was 58 weeks. |
||||||
|
2Placebo patients in the 2 Phase III trials in Crohn's disease received an initial dose of 5 mg/kg REMICADE at study start and were on placebo in the maintenance phase. Patients who were randomized to the placebo maintenance group and then later crossed over to REMICADE are included in the REMICADE group in ALT analysis. Median follow-up was 54 weeks. |
||||||
|
3Median follow-up was 30 weeks. Specifically, the median duration of follow-up was 30 weeks for placebo and 31 weeks for REMICADE. |
||||||
|
4Median follow-up was 24 weeks for placebo group and 102 weeks for REMICADE group. |
||||||
|
5Median follow-up was 39 weeks for REMICADE group and 18 weeks for placebo group. |
||||||
|
6ALT values are obtained in 2 Phase 3 psoriasis studies with median follow-up of 50 weeks for REMICADE and 16 weeks for placebo. |
||||||
| Proportion of patients with elevated ALT | ||||||
| >1 to <3 x ULN | ≥3 x ULN | ≥5 x ULN | ||||
| Placebo | REMICADE | Placebo | REMICADE | Placebo | REMICADE | |
| Rheumatoid arthritis1 | 24% | 34% | 3% | 4% | <1% | <1% |
| Crohn's disease2 | 34% | 39% | 4% | 5% | 0% | 2% |
| Ulcerative colitis3 | 12% | 17% | 1% | 2% | <1% | <1% |
| Ankylosing spondylitis4 | 15% | 51% | 0% | 10% | 0% | 4% |
| Psoriatic arthritis5 | 16% | 50% | 0% | 7% | 0% | 2% |
| Plaque psoriasis6 | 24% | 49% | <1% | 8% | 0% | 3% |
Adverse Reactions in Pediatric Crohn's Disease
There were some differences in the adverse reactions observed in the pediatric patients receiving REMICADE compared to those observed in adults with Crohn's disease. These differences are discussed in the following paragraphs.
The following adverse events were reported more commonly in 103 randomized pediatric Crohn's disease patients administered 5 mg/kg REMICADE through 54 weeks than in 385 adult Crohn's disease patients receiving a similar treatment regimen: anemia (11%), blood in stool (10%), leukopenia (9%), flushing (9%), viral infection (8%), neutropenia (7%), bone fracture (7%), bacterial infection (6%), and respiratory tract allergic reaction (6%).
Infections were reported in 56% of randomized pediatric patients in Study Peds Crohn's and in 50% of adult patients in Study Crohn's I. In Study Peds Crohn's, infections were reported more frequently for patients who received every 8 week as opposed to every 12 week infusions (74% and 38%, respectively), while serious infections were reported for 3 patients in the every 8 week and 4 patients in the every 12 week maintenance treatment group. The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was abscess. Pneumonia was reported for 3 patients, (2 in the every 8 week and 1 in the every 12 week maintenance treatment groups). Herpes zoster was reported for 2 patients in the every 8 week maintenance treatment group.
In Study Peds Crohn's, 18% of randomized patients experienced one or more infusion reactions, with no notable difference between treatment groups. Of the 112 patients in Study Peds Crohn's, there were no serious infusion reactions, and 2 patients had non-serious anaphylactoid reactions.
Antibodies to REMICADE developed in 3% of pediatric patients in Study Peds Crohn's.
Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 18% of pediatric patients in Crohn's disease clinical trials; 4% had ALT elevations ≥ 3 x ULN, and 1% had elevations ≥ 5 x ULN. (Median follow-up was 53 weeks.)
Adverse Reactions in Psoriasis Studies
During the placebo-controlled portion across the three clinical trials up to week 16, the proportion of patients who experienced at least 1 SAE (defined as resulting in death, life threatening, requires hospitalization, or persistent or significant disability/incapacity) was 1.7% in the 3 mg/kg REMICADE group, 3.2% in the placebo group, and 3.9% in the 5 mg/kg REMICADE group.
Among patients in the 2 Phase 3 studies, 12.4% of patients receiving REMICADE 5 mg/kg every 8 weeks through one year of maintenance treatment experienced at least 1 SAE in Study I. In Study II, 4.1% and 4.7% of patients receiving REMICADE 3 mg/kg and 5 mg/kg every 8 weeks, respectively, through one year of maintenance treatment experienced at least 1 SAE.
One death due to bacterial sepsis occurred 25 days after the second infusion of 5 mg/kg REMICADE. Serious infections included sepsis, and abscesses. In Study I, 2.7% of patients receiving REMICADE 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 serious infection. In Study II, 1.0% and 1.3% of patients receiving REMICADE 3 mg/kg and 5 mg/kg, respectively, through 1 year of treatment experienced at least 1 serious infection. The most common serious infection (requiring hospitalization) were abscesses (skin, throat, and peri-rectal) reported by 5 (0.7%) patients in the 5 mg/kg REMICADE group. Two active cases of tuberculosis were reported: 6 weeks and 34 weeks after starting REMICADE.
In placebo-controlled portion of the psoriasis studies, 7 of 1123 patients who received REMICADE at any dose were diagnosed with at least one NMSC compared to 0 of 334 patients who received placebo.
In the psoriasis studies, 1% (15/1373) of patients experienced serum sickness or a combination of arthralgia and/or myalgia with fever, and/or rash, usually early in the treatment course. Of these patients, 6 required hospitalization due to fever, severe myalgia, arthralgia, swollen joints, and immobility.
Other Adverse Reactions
Safety data are available from 4779 REMICADE-treated adult patients, including 1304 with rheumatoid arthritis, 1106 with Crohn's disease, 484 with ulcerative colitis, 202 with ankylosing spondylitis, 293 with psoriatic arthritis, 1373 with plaque psoriasis and 17 with other conditions. (For information on other adverse reactions in pediatric patients, see ADVERSE REACTIONS – Adverse Reactions in Pediatric Crohn's Disease). Adverse events reported in ≥5% of all patients with rheumatoid arthritis receiving 4 or more infusions are in Table 12. The types and frequencies of adverse reactions observed were similar in REMICADE-treated rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis and Crohn's disease patients except for abdominal pain, which occurred in 26% of REMICADE-treated patients with Crohn's disease. In the Crohn's disease studies, there were insufficient numbers and duration of follow-up for patients who never received REMICADE to provide meaningful comparisons.
| Placebo | REMICADE | |
| (n=350) | (n=1129) | |
| Average weeks of follow-up | 59 | 66 |
| Gastrointestinal | ||
| Nausea | 20% | 21% |
| Abdominal Pain | 8% | 12% |
| Diarrhea | 12% | 12% |
| Dyspepsia | 7% | 10% |
| Respiratory | ||
| Upper respiratory tract infection | 25% | 32% |
| Sinusitis | 8% | 14% |
| Pharyngitis | 8% | 12% |
| Coughing | 8% | 12% |
| Bronchitis | 9% | 10% |
| Rhinitis | 5% | 8% |
| Skin and appendages disorders | ||
| Rash | 5% | 10% |
| Pruritus | 2% | 7% |
| Body as a whole-general disorders | ||
| Fatigue | 7% | 9% |
| Pain | 7% | 8% |
| Resistance mechanism disorders | ||
| Fever | 4% | 7% |
| Moniliasis | 3% | 5% |
| Central and peripheral nervous system disorders | ||
| Headache | 14% | 18% |
| Musculoskeletal system disorders | ||
| Back pain | 5% | 8% |
| Arthralgia | 7% | 8% |
| Urinary system disorders | ||
| Urinary tract infection | 6% | 8% |
| Cardiovascular disorders, general | ||
| Hypertension | 5% | 7% |
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not predict the rates observed in broader patient populations in clinical practice.
The most common serious adverse events observed in clinical trials were infections (see ADVERSE REACTIONS, Infections). Other serious, medically relevant adverse events ≥0.2% or clinically significant adverse events by body system were as follows:
Body as a whole: allergic reaction, diaphragmatic hernia, edema, surgical/procedural sequela
Blood: pancytopenia
Cardiovascular: circulatory failure, hypotension, syncope
Gastrointestinal: constipation, gastrointestinal hemorrhage, ileus, intestinal obstruction, intestinal perforation, intestinal stenosis, pancreatitis, peritonitis, proctalgia
Central & Peripheral Nervous: meningitis, neuritis, peripheral neuropathy, dizziness
Heart Rate and Rhythm: arrhythmia, bradycardia, cardiac arrest, tachycardia
Liver and Biliary: biliary pain, cholecystitis, cholelithiasis, hepatitis
Metabolic and Nutritional: dehydration
Musculoskeletal: intervertebral disk herniation, tendon disorder
Myo-, Endo-, Pericardial and Coronary Valve: myocardial infarction
Platelet, Bleeding and Clotting: thrombocytopenia
Neoplasms: basal cell, breast, lymphoma
Psychiatric: confusion, suicide attempt
Red Blood Cell: anemia, hemolytic anemia
Reproductive: menstrual irregularity
Resistance Mechanism: cellulitis, sepsis, serum sickness
Respiratory: adult respiratory distress syndrome, lower respiratory tract infection (including pneumonia), pleural effusion, pleurisy, pulmonary edema, respiratory insufficiency
Skin and Appendages: increased sweating, ulceration
Urinary: renal calculus, renal failure
Vascular (Extracardiac): brain infarction, pulmonary embolism, thrombophlebitis
White Cell and Reticuloendothelial: leukopenia, lymphadenopathy
Post-marketing Adverse Events
The following adverse events, some with fatal outcomes, have been reported during post-approval use of REMICADE: neutropenia (see WARNINGS, Hematologic Events), interstitial lung disease (including pulmonary fibrosis/interstitial pneumonitis and very rare rapidly progressive disease), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, pericardial effusion, systemic and cutaneous vasculitis, erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, Guillain-Barré syndrome, psoriasis (including new onset and pustular, primarily palmar/plantar), transverse myelitis, and neuropathies (additional neurologic events have also been observed, see WARNINGS, Neurologic Events) and acute liver failure, jaundice, hepatitis, and cholestasis (see WARNINGS, Hepatotoxicity). Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to REMICADE exposure.
The following serious adverse events have been reported in the post-marketing experience in children: infections (some fatal) including opportunistic infections and tuberculosis, infusion reactions, and hypersensitivity reactions.
Serious adverse events in the post-marketing experience with REMICADE in the pediatric population have also included malignancies, including hepatosplenic T-cell lymphomas (see Boxed WARNINGS and WARNINGS), transient hepatic enzyme abnormalities, lupus-like syndromes, and the development of autoantibodies.
OVERDOSAGE
Single doses up to 20 mg/kg have been administered without any direct toxic effect. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
DOSAGE AND ADMINISTRATION
Rheumatoid Arthritis
The recommended dose of REMICADE is 3 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion then every 8 weeks thereafter. REMICADE should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses (see ADVERSE REACTIONS, Infections).
Crohn's Disease or Fistulizing Crohn's Disease
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adults with moderately to severely active Crohn's disease or fistulizing disease. For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue REMICADE in these patients.
The recommended dose of REMICADE for children with moderately to severely active Crohn's disease is 5 mg/kg given as an induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
Ankylosing Spondylitis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion, then every 6 weeks thereafter.
Psoriatic Arthritis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion then every 8 weeks thereafter. REMICADE can be used with or without methotrexate.
Plaque Psoriasis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion, followed by additional doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter.
Ulcerative Colitis
The recommended dose of REMICADE is 5 mg/kg given as an induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active ulcerative colitis.
Administration Instructions Regarding Infusion Reactions
Adverse effects during administration of REMICADE have included flu-like symptoms, headache, dyspnea, hypotension, transient fever, chills, gastrointestinal symptoms, and skin rashes. Anaphylaxis might occur at any time during REMICADE infusion. Approximately 20% of REMICADE-treated patients in all clinical trials experienced an infusion reaction compared with 10% of placebo-treated patients (see ADVERSE REACTIONS, Infusion-related Reactions). Prior to infusion with REMICADE, premedication may be administered at the physician's discretion. Premedication could include antihistamines (anti-H1 +/- anti-H2), acetaminophen and/or corticosteroids.
During infusion, mild to moderate infusion reactions may improve following slowing or suspension of the infusion, and upon resolution of the reaction, reinitiation at a lower infusion rate and/or therapeutic administration of antihistamines, acetaminophen, and/or corticosteroids. For patients that do not tolerate the infusion following these interventions, REMICADE should be discontinued.
During or following infusion, patients that have severe infusion-related hypersensitivity reactions should be discontinued from further REMICADE treatment. The management of severe infusion reactions should be dictated by the signs and symptoms of the reaction. Appropriate personnel and medication should be available to treat anaphylaxis if it occurs.
Preparation and Administration Instructions
Use aseptic technique.
REMICADE vials do not contain antibacterial preservatives. Therefore, the vials after reconstitution should be used immediately, not re-entered or stored. The diluent to be used for reconstitution is 10 mL of Sterile Water for Injection, USP. The total dose of the reconstituted product must be further diluted to 250 mL with 0.9% Sodium Chloride Injection, USP. The infusion concentration should range between 0.4 mg/mL and 4 mg/mL. The REMICADE infusion should begin within 3 hours of preparation.
- Calculate the dose and the number of REMICADE vials needed. Each REMICADE vial contains 100 mg of infliximab. Calculate the total volume of reconstituted REMICADE solution required.
- Reconstitute each REMICADE vial with 10 mL of Sterile Water for Injection, USP, using a syringe equipped with a 21-gauge or smaller needle. Remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of Sterile Water for Injection, USP, to the glass wall of the vial. Gently swirl the solution by rotating the vial to dissolve the lyophilized powder. Avoid prolonged or vigorous agitation. DO NOT SHAKE. Foaming of the solution on reconstitution is not unusual. Allow the reconstituted solution to stand for 5 minutes. The solution should be colorless to light yellow and opalescent, and the solution may develop a few translucent particles as infliximab is a protein. Do not use if opaque particles, discoloration, or other foreign particles are present.
- Dilute the total volume of the reconstituted REMICADE solution dose to 250 mL with 0.9% Sodium Chloride Injection, USP, by withdrawing a volume of 0.9% Sodium Chloride Injection, USP, equal to the volume of reconstituted REMICADE from the 0.9% Sodium Chloride Injection, USP, 250 mL bottle or bag. Slowly add the total volume of reconstituted REMICADE solution to the 250 mL infusion bottle or bag. Gently mix.
- The infusion solution must be administered over a period of not less than 2 hours and must use an infusion set with an in-line, sterile, non-pyrogenic, low-protein-binding filter (pore size of 1.2 μm or less). Any unused portion of the infusion solution should not be stored for reuse.
- No physical biochemical compatibility studies have been conducted to evaluate the co-administration of REMICADE with other agents. REMICADE should not be infused concomitantly in the same intravenous line with other agents.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.
Storage
Store the lyophilized product under refrigeration at 2°C to 8°C (36°F to 46°F). Do not use beyond the expiration date. This product contains no preservative.
HOW SUPPLIED
REMICADE lyophilized concentrate for IV injection is supplied in individually-boxed single-use vials in the following strength:
NDC 57894-030-01 100 mg infliximab in a 20 mL vial
REFERENCES
- American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
- See latest Center for Disease Control guidelines and recommendations for tuberculosis testing in immunocompromised patients.
- Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Molec Immunol 1993;30:1443-1453.
- Scallon BJ, Moore MA, Trinh H, et al. Chimeric anti-TNFα monoclonal antibody cA2 binds recombinant transmembrane TNFα and activates immune effector functions. Cytokine 1995;7:251-259.
- ten Hove T, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 2002;50:206-211.
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41(9):1552-1563.
- Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) vs. placebo in rheumatoid arthritis. Lancet 1994;344(8930):1105-1110.
- Van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective follow-up of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35(1):26-34.
- Targan SR, Hanauer SB, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med 1997;337(15):1029-1035.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomized trial. Lancet 2002; 359:1541-1549.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398-1405.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361-368.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625-1629.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumor necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management.Lancet Infect Dis 2003;3:148-155.
- Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic γδT-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 2003;102(13):4261-4269
©Centocor, Inc. 2006
Malvern, PA 19355, USA
1-800-457-6399
License #1242
May 2008
Rx Only
Medication Guide
REMICADE® (Rem-eh-kaid)
(infliximab)
Read the Medication Guide that comes with REMICADE before you receive the first treatment, and before each time you get a treatment of REMICADE. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment.
What is the most important information I should know about REMICADE?
REMICADE is a medicine that affects your immune system. It can cause serious side effects including:
Serious Infections
- Patients treated with REMICADE and other medicines that block TNF have an increased risk for infections. Some patients have had serious infections while receiving REMICADE. In some cases, the infections got worse (progressed) and became serious enough that patients needed to be in the hospital for treatment. These serious infections include TB (tuberculosis), and infections caused by viruses, fungi or bacteria that have spread throughout the body. Some patients have died from these infections.
- Tell your doctor right away if you have any of the following symptoms, which may be early signs of a serious infection, while taking or after taking REMICADE:
- a fever
- feel very tired
- have a cough
- have flu-like symptoms
- warm, red, or painful skin
These may be early signs of a serious infection.
Cancer
- Some children and young adults with Crohn's disease who have received REMICADE have developed a rare type of cancer called Hepatosplenic T-cell Lymphoma. This type of cancer often results in death. These patients were also receiving drugs known as azathioprine or 6-mercaptopurine.
- Tell your doctor if you have ever had any type of cancer.
See also, " What are the possible side effects of REMICADE?" below.
What is REMICADE?
REMICADE is a prescription medicine that is approved for patients with:
- Rheumatoid Arthritis - adults with moderately to severely active rheumatoid arthritis, along with the medicine methotrexate
- Crohn's Disease - children over the age of 6 and adults with Crohn's disease who have not responded well enough to other medicines
- Ankylosing Spondylitis
- Psoriatic Arthritis
- Plaque Psoriasis - adult patients with plaque psoriasis that is chronic (doesn't go away) severe, extensive, and/or disabling.
- Ulcerative Colitis - adult patients with moderately to severely active ulcerative colitis who have not responded well enough to other medicines.
REMICADE blocks the action of a protein in your body called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha is made by your body's immune system. People with certain diseases have too much TNF-alpha that can cause the immune system to attack normal healthy parts of the body. REMICADE can block the damage caused by too much TNF-alpha.
Who should not receive REMICADE?
You should not receive REMICADE if you have:
- heart failure, unless your doctor has examined you and decided that you are able to take REMICADE. Talk to your doctor about your heart failure.
- had an allergic reaction to REMICADE, or any of the other ingredients in REMICADE. See the end of this Medication Guide for a complete list of ingredients in REMICADE.
What should I tell my doctor before starting treatment with REMICADE?
Your doctor will assess your health before each treatment.
Tell your doctor about all of your medical conditions, including if you:
- have any kind of infection even if it is very minor (such as an open cut or sore). REMICADE affects the body's immune system and makes you less able to fight infections.
- have an infection that won't go away or a history of infection that keeps coming back.
- have had TB (tuberculosis), or if you have recently been near anyone who might have TB. If you have been near someone with TB and have the TB germ in your body, even if you don't have symptoms of an infection, you can get a serious TB infection while taking REMICADE. Sometimes these serious TB infections can cause death.
- were born in, lived in or traveled to countries where there is more risk for getting TB. Ask your doctor if you are not sure.
- live or have lived in certain parts of the country where there is more risk for certain kinds of fungal infections (histoplasmosis or coccidioidomycosis). These infections may develop or become more severe if you take REMICADE. If you don't know if you have lived in an area where histoplasmosis or coccidioidomycosis is common, ask your doctor.
- have or had hepatitis B. If you are a chronic carrier of the virus that causes hepatitis B, taking REMICADE could cause the hepatitis B virus to become an active infection again.
- have other liver problems including liver failure.
- have heart failure or other heart conditions. If you have heart failure, it may get worse while you take REMICADE.
- have or have had any type of cancer.
- have had phototherapy (treatment with ultraviolet light or sunlight along with a medicine to make your skin sensitive to light) for psoriasis. You may have a higher chance of getting skin cancer while receiving REMICADE.
- have COPD (Chronic Obstructive Pulmonary Disease), a specific type of lung disease. Patients with COPD may have an increased risk of getting cancer while taking REMICADE.
- have or have had a condition that affects your nervous system such as
- multiple sclerosis, or Guillain-Barré syndrome, or
- if you experience any numbness or tingling, or
- if you have had a seizure.
- have recently received or are scheduled to receive a vaccine. Adults and children should not receive a live vaccine while taking REMICADE. Children with Crohn's disease should have all of their vaccines brought up to date before starting treatment with REMICADE.
- are pregnant or planning to become pregnant. It is not known if REMICADE harms your unborn baby. REMICADE should be given to a pregnant woman only if clearly needed. Talk to your doctor about stopping REMICADE if you are pregnant or planning to become pregnant.
- are breast-feeding or planning to breast-feed. It is not known whether REMICADE passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking REMICADE. You should not breast-feed while taking REMICADE.
How should I receive REMICADE?
- You will be given REMICADE through a needle placed in a vein (IV or intravenous infusion) in your arm.
- Your doctor may decide to give you medicine before starting the REMICADE infusion to prevent or lessen side effects.
- Only a healthcare professional should prepare the medicine and administer it to you.
- REMICADE will be given to you over a period of about 2 hours.
- If you have side effects from REMICADE, the infusion may need to be adjusted or stopped. In addition, your healthcare professional may decide to treat your symptoms.
- A healthcare professional will monitor you during the REMICADE infusion and for a period of time afterward for side effects.
- Your doctor may do certain tests while you are taking REMICADE to monitor you for side effects and to see how well you respond to the treatment.
- Your doctor will determine the right dose of REMICADE for you and how often you should receive it. Make sure to discuss with your doctor when you will receive infusions and to come in for all your infusions and follow-up appointments.
What should I avoid while receiving REMICADE?
Do not take REMICADE and the medication KINERET (Anakinra) together.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines and show them to your doctor and pharmacist when you get a new medicine.
What are the possible side effects of REMICADE?
Serious and sometimes fatal side effects have been reported in patients taking REMICADE (see also " What is the most important information I should know about REMICADE?"). These include:
Serious Infections
- Some patients have had serious infections while receiving REMICADE. These serious infections include tuberculosis (TB) and infections caused by viruses, fungi, or bacteria that have spread throughout the body. Some patients die from these infections. If you get an infection while receiving treatment with REMICADE your doctor will treat your infection and may need to stop your REMICADE treatment.
- Tell your doctor right away if you have any of the following signs of an infection while taking or after taking REMICADE:
- a fever
- feel very tired
- have a cough
- have flu-like symptoms
- warm, red, or painful skin
- Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with REMICADE and during treatment with REMICADE.
- Even if your TB test is negative your doctor should carefully monitor you for TB infections while you are taking REMICADE. Patients who had a negative TB skin test before receiving REMICADE have developed active TB.
- If you are a chronic carrier of the hepatitis B virus, the virus can become active while you are being treated with REMICADE. In some cases patients have died as a result of hepatitis B virus being reactivated. Your doctor may do a blood test before you start treatment with REMICADE and occasionally while you are being treated. Tell your doctor if you have any of the following symptoms:
- feel unwell
- poor appetite
- tiredness (fatigue)
- fever, skin rash and/or joint pain
Cancer
- In clinical studies, more cancers were seen in patients who took REMICADE and other medicines that block TNF than patients who did not receive these treatments.
- Some children and young adults with Crohn's disease who have received REMICADE have developed a rare type of cancer called Hepatosplenic T-cell Lymphoma.. This type of cancer often results in death. These patients were also receiving drugs known as azathioprine or 6-mercaptopurine.
- People who have been treated for rheumatoid arthritis, Crohn's disease, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis for a long time may be more likely to develop lymphoma. This is especially true for people with very active disease.
- Patients with COPD (a specific type of lung disease) may have an increased risk for getting cancer while being treated with REMICADE.
- If you take REMICADE, your chances of getting lymphoma or other cancers may increase.
Heart Failure
If you have a heart problem called congestive heart failure, your doctor should check you closely while you are taking REMICADE. Your congestive heart failure may get worse while you are taking REMICADE. Be sure to tell your doctor of any new or worse symptoms including:
- Shortness of breath
- Swelling of ankles or feet
- Sudden weight gain
Treatment with REMICADE may need to be stopped if you get new or worse congestive heart failure.
Liver Injury
In rare cases, some patients taking REMICADE have developed serious liver problems. Tell your doctor if you have
- Jaundice (skin and eyes turning yellow)
- Dark brown-colored urine
- Pain on the right side of your stomach area (right-sided abdominal pain)
- Fever
- Extreme tiredness (severe fatigue)
Blood Problems
In some patients taking REMICADE, the body may not make enough of the blood cells that help fight infections or help stop bleeding. Tell your doctor if you
- Have a fever that does not go away
- Bruise or bleed very easily
- Look very pale
Nervous System Disorders
In rare cases, patients taking REMICADE have developed problems with their nervous system. Tell your doctor if you have
- Changes in your vision
- Weakness in your arms and/or legs
- Numbness or tingling in any part of your body
- Seizures
Allergic Reactions
Some patients have had allergic reactions to REMICADE. Some of these reactions were severe. These reactions can happen while you are getting your REMICADE treatment or shortly afterwards. Your doctor may need to stop or pause your treatment with REMICADE and may give you medicines to treat the allergic reaction. Signs of an allergic reaction can include:
- Hives (red, raised, itchy patches of skin)
- Difficulty breathing
- Chest pain
- High or low blood pressure
- Fever
- Chills
Some patients treated with REMICADE have had delayed allergic reactions. The delayed reactions occurred 3 to 12 days after receiving treatment with REMICADE. Tell your doctor right away if you have any of these signs of delayed allergic reaction to REMICADE:
- Fever
- Rash
- Headache
- Sore throat
- Muscle or joint pain
- Swelling of the face and hands
- Difficulty swallowing
Lupus-like Syndrome
Some patients have developed symptoms that are like the symptoms of Lupus. If you develop any of the following symptoms your doctor may decide to stop your treatment with REMICADE.
- Chest discomfort or pain that does not go away
- Shortness of breath
- Joint pain
- Rash on the cheeks or arms that gets worse in sun
The most common side effects of REMICADE are
- Respiratory infections, such as sinus infections and sore throat)
- Headache
- Rash
- Coughing
- Stomach pain
Children who took REMICADE in studies for Crohn's disease, showed some differences in side effects compared with adults who took REMICADE for Crohn's disease. The side effects that happened more in children were: anemia (low red blood cells), blood in stool, leukopenia (low white blood cells), flushing (redness or blushing), viral infections, neutropenia (low neutrophils, the white blood cells that fight infection), bone fracture, bacterial infection and allergic reactions of the breathing tract.
Tell your doctor about any side effect that bothers you or does not go away.
These are not all of the side effects with REMICADE. Ask your doctor or pharmacist for more information.
General information about REMICADE
Medicines are sometimes prescribed for purposes that are not mentioned in Medication Guides or patient information sheets. Do not use REMICADE for a condition for which it was not prescribed.
This information sheet summarizes the most important information about REMICADE. You can ask your doctor or pharmacist for information about REMICADE that is written for health professionals.
For more information go to www.remicade.com or call 1-800-457-6399.
What are the ingredients in REMICADE?
The active ingredient is Infliximab.
The inactive ingredients in REMICADE include: sucrose, polysorbate 80, monobasic sodium phosphate monohydrate, and dibasic sodium phosphate dihydrate. No Preservatives are present.
Product developed and manufactured by:
Centocor, Inc.
200 Great Valley Parkway
Malvern, PA 19355
Revised May 2008
This Medication Guide has been approved by the U.S. Food and Drug Administration.
| REMICADE (infliximab) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 06/2008Centocor, Inc.