|
|
||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: POTENTIAL LIVER INJURY
LETAIRIS (ambrisentan) can cause elevation of liver aminotransferases (ALT and AST) to at least 3 times the upper limit of normal (ULN). LETAIRIS treatment was associated with aminotransferase elevations >3 x ULN in 0.8% of patients in 12-week trials and 2.8% of patients including long-term open-label trials out to one year. One case of aminotransferase elevations >3 x ULN has been accompanied by bilirubin elevations >2 x ULN. Because these changes are a marker for potentially serious liver injury, serum aminotransferase levels (and bilirubin if aminotransferase levels are elevated) must be measured prior to initiation of treatment and then monthly.
In the post-marketing period with another endothelin receptor antagonist (ERA), bosentan, rare cases of unexplained hepatic cirrhosis were reported after prolonged (>12 months) therapy. In at least one case with bosentan, a late presentation (after >20 months of treatment) included pronounced elevations in aminotransferases and bilirubin levels accompanied by non-specific symptoms, all of which resolved slowly over time after discontinuation of the suspect drug. This case reinforces the importance of strict adherence to the monthly monitoring schedule for the duration of treatment.
Elevations in aminotransferases require close attention. LETAIRIS should generally be avoided in patients with elevated aminotransferases (>3 x ULN) at baseline because monitoring liver injury may be more difficult. If liver aminotransferase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin >2 x ULN, treatment should be stopped. There is no experience with the re-introduction of LETAIRIS in these circumstances.
CONTRAINDICATION: PREGNANCY
LETAIRIS is very likely to produce serious birth defects if used by pregnant women, as this effect has been seen consistently when it is administered to animals [see Contraindications ( 4.1)]. Pregnancy must therefore be excluded before the initiation of treatment with LETAIRIS and prevented thereafter by the use of at least two reliable methods of contraception unless the patient has had a tubal sterilization or Copper T 380A IUD or LNg 20 IUD inserted, in which case no other contraception is needed. Obtain monthly pregnancy tests.
Because of the risks of liver injury and birth defects, LETAIRIS is available only through a special restricted distribution program called the LETAIRIS Education and Access Program (LEAP), by calling 1-866-664-LEAP (5327). Only prescribers and pharmacies registered with LEAP may prescribe and distribute LETAIRIS. In addition, LETAIRIS may be dispensed only to patients who are enrolled in and meet all conditions of LEAP [see WARNINGS, Prescribing and Distribution Program for LETAIRIS].
1 INDICATIONS AND USAGE
LETAIRIS is indicated for the treatment of pulmonary arterial hypertension (WHO Group 1) in patients with WHO class II or III symptoms to improve exercise capacity and delay clinical worsening.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Dosage
Initiate treatment at 5 mg once daily with or without food, and consider increasing the dose to 10 mg once daily if 5 mg is tolerated.
Tablets may be administered with or without food. Tablets should not be split, crushed, or chewed. Doses higher than 10 mg once daily have not been studied in patients with pulmonary arterial hypertension (PAH). Liver function tests should be measured prior to initiation and during treatment with LETAIRIS [see Warnings and Precautions ( 5.1)].
2.2 Women of Childbearing Potential
Treat women of childbearing potential only after a negative pregnancy test and treat only women who are using two reliable methods of contraception unless the patient has had a tubal sterilization or a Copper T 380A IUD or LNg 20 IUD inserted. In those cases, no other contraception is needed. Pregnancy tests should be obtained monthly in women of childbearing potential taking LETAIRIS [see Contraindications ( 4.1)].
2.3 Pre-existing Hepatic Impairment
LETAIRIS is not recommended in patients with moderate or severe hepatic impairment [see Special Populations ( 8.7)]. Use caution in patients with mild hepatic impairment.
3 DOSAGE FORMS AND STRENGTHS
LETAIRIS is available as 5 mg and 10 mg film-coated, unscored tablets.
4 CONTRAINDICATIONS
4.1 Pregnancy Category X
LETAIRIS may cause fetal harm when administered to a pregnant woman. Ambrisentan was teratogenic at oral doses of ≥15 mg/kg/day in rats and ≥7 mg/kg/day in rabbits; it was not studied at lower doses. In both species, there were abnormalities of the lower jaw and hard and soft palate, malformation of the heart and great vessels, and failure of formation of the thymus and thyroid. Teratogenicity is a class effect of endothelin receptor antagonists. There are no data on the use of LETAIRIS in pregnant women.
LETAIRIS is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Pregnancy must be excluded before the initiation of treatment with LETAIRIS and prevented thereafter by the use of two reliable methods of contraception [see Dosage and Administration ( 2.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Potential Liver Injury
(SEE BOXED WARNING)
Treatment with endothelin receptor antagonists has been associated with dose-dependent liver injury manifested primarily by elevation of serum aminotransferases (ALT or AST), but sometimes accompanied by abnormal liver function (elevated bilirubin). The combination of aminotransferases greater than 3-times the upper limit of normal (>3 x ULN) and total bilirubin >2 x ULN is a marker for potentially serious hepatic injury.
Liver function tests were closely monitored in all clinical studies with LETAIRIS. For all LETAIRIS-treated patients (N=483), the 12-week incidence of aminotransferases >3 x ULN was 0.8% and >8 x ULN was 0.2%. For placebo-treated patients, the 12-week incidence of aminotransferases >3 x ULN was 2.3% and >8 x ULN was 0.0%. The 1-year rate of aminotransferase elevations >3 x ULN with LETAIRIS was 2.8% and >8 x ULN was 0.5%. One case of aminotransferase elevations >3 x ULN has been accompanied by bilirubin elevations >2 x ULN.
Liver chemistries must be measured prior to initiation of LETAIRIS and at least every month thereafter. If there are aminotransferase elevations >3 x ULN and ≤5 x ULN, they should be re-measured. If the confirmed level is >3 x ULN and ≤5 x ULN, reduce the daily dose or interrupt treatment and continue to monitor every two weeks until the levels are <3 x ULN. If there are aminotransferase elevations >5 x ULN and ≤8 x ULN, LETAIRIS should be discontinued and monitoring should continue until the levels are <3 x ULN. LETAIRIS can then be re-initiated with more frequent measurement of aminotransferase levels. If there are aminotransferase elevations >8 x ULN, treatment should be stopped and re-initiation should not be considered.
LETAIRIS is not recommended in patients with elevated aminotransferases (>3 x ULN) at baseline because monitoring liver injury may be more difficult. If aminotransferase elevations are accompanied by clinical symptoms of liver injury (such as anorexia, nausea, vomiting, fever, malaise, fatigue, right upper quadrant abdominal discomfort, itching, or jaundice) or increases in bilirubin >2 x ULN, LETAIRIS treatment should be stopped. There is no experience with the re-introduction of LETAIRIS in these circumstances.
5.2 Hematological Changes
Decreases in hemoglobin concentration and hematocrit have followed administration of other endothelin receptor antagonists and were observed in clinical studies with LETAIRIS. These decreases were observed within the first few weeks of treatment with LETAIRIS, and stabilized thereafter. The mean decrease in hemoglobin from baseline to end of treatment for those patients receiving LETAIRIS in the 12-week placebo-controlled studies was 0.8 g/dL.
Marked decreases in hemoglobin (>15% decrease from baseline resulting in a value below the lower limit of normal) were observed in 7% of all patients receiving LETAIRIS (and 10% of patients receiving 10 mg) compared to 4% of patients receiving placebo. The cause of the decrease in hemoglobin is unknown, but it does not appear to result from hemorrhage or hemolysis.
Hemoglobin must be measured prior to initiation of LETAIRIS and should be measured at one month and periodically thereafter. If a clinically significant decrease in hemoglobin is observed and other causes have been excluded, discontinuation of treatment should be considered.
5.3 Fluid Retention
Peripheral edema is a known class effect of endothelin receptor antagonists, and is also a clinical consequence of PAH and worsening PAH. In the placebo-controlled studies, there was an increased incidence of peripheral edema in patients treated with doses of 5 or 10 mg LETAIRIS compared to placebo [see Adverse Reactions ( 6)]. Most edema was mild to moderate in severity, and it occurred with greater frequency and severity in elderly patients.
In addition, there have been post-marketing reports of fluid retention in patients with pulmonary hypertension, occurring within weeks after starting LETAIRIS. Patients required intervention with a diuretic, fluid management, or, in some cases, hospitalization for decompensating heart failure.
If clinically significant fluid retention develops, with or without associated weight gain, further evaluation should be undertaken to determine the cause, such as LETAIRIS or underlying heart failure, and the possible need for specific treatment or discontinuation of LETAIRIS therapy.
5.4 Co-administration of LETAIRIS and Cyclosporine A
Cyclosporine is a strong inhibitor of P-glycoprotein (P-gp), Organic Anion Transport Protein (OATP), and CYP3A4. In vitro data indicate ambrisentan is a substrate of P-gp, OATP and CYP3A. Therefore, use caution when LETAIRIS is co-administered with cyclosporine A because cyclosporine A may cause increased exposure to LETAIRIS [see Drug Interactions ( 7)].
5.5 Co-administration of LETAIRIS and Strong CYP3A and 2C19 Inhibitors
Use caution when LETAIRIS is co-administered with strong CYP3A-inhibitors (e.g., ketoconazole) and CYP2C19-inhibitors (e.g., omeprazole) [see Drug Interactions ( 7)].
5.6 Prescribing and Distribution Program for LETAIRIS
Because of the risks of liver injury and birth defects, LETAIRIS is available only through a special restricted distribution program called the LETAIRIS Education and Access Program (LEAP). Only prescribers and pharmacies registered with LEAP may prescribe and distribute LETAIRIS. In addition, LETAIRIS may be dispensed only to patients who are enrolled in and meet all conditions of LEAP.
To enroll in LEAP, prescribers must complete the LEAP Prescriber Enrollment and Agreement Form indicating agreement to (see LEAP Prescriber Enrollment and Agreement Form for full prescribing physician agreement):
- Read the Prescribing Information (PI) and Medication Guide for LETAIRIS.
- Enroll all patients in LEAP and re-enroll patients after the first 6 months of treatment and annually thereafter.
- Review the LETAIRIS Medication Guide and patient education brochure(s) with every patient.
- Educate patients on the risks of LETAIRIS, including the risks of hepatotoxicity and teratogenicity [see Boxed Warning].
- Educate and counsel women of childbearing potential to use two different forms of contraception including at least one primary form during LETAIRIS treatment and for one month following treatment discontinuation. If the patient has had a tubal sterilization or a Copper T 380A IUD or LNg 20 IUD inserted, no additional contraception is needed [see Boxed Warning, Contraindication ( 4.1)].
Primary forms of contraception include tubal sterilization, hormonal (combination oral contraceptives, transdermal patch, injectables, implantables, or vaginal ring), IUD, and a partner's vasectomy. A Copper T 380A IUD or LNg 20 IUD can be used alone, i.e. without a secondary form of contraception, as can tubal sterilization.
Secondary forms of contraception include barrier contraceptives such as latex condoms, diaphragms, and cervical caps. - Order and review liver function tests (including aminotransferases and bilirubin) prior to initiation of LETAIRIS treatment and monthly during treatment.
- For women of childbearing potential, order and review a pregnancy test prior to initiation of LETAIRIS treatment and monthly during treatment.
- Counsel patients who fail to comply with the program requirements.
- Notify LEAP of any adverse events, including liver injury, or if any patient becomes pregnant during LETAIRIS treatment.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety data for LETAIRIS were obtained from two 12-week, placebo-controlled studies in patients with PAH (ARIES-1 and ARIES-2) and four nonplacebo-controlled studies in 483 patients with PAH who were treated with doses of 1, 2.5, 5, or 10 mg once daily. The exposure to LETAIRIS in these studies ranged from 1 day to 4 years (N=418 for at least 6 months and N=343 for at least 1 year).
In ARIES-1 and ARIES-2, a total of 261 patients received LETAIRIS at doses of 2.5, 5, or 10 mg once daily and 132 patients received placebo. The adverse events that occurred in >3% of the patients receiving LETAIRIS and were more frequent on LETAIRIS than placebo are shown in Table 1.
| Placebo (N=132) | LETAIRIS (N=261) |
||
|---|---|---|---|
| Adverse event | n (%) | n (%) | Placebo-adjusted (%) |
|
Note: This table includes all adverse events >3% incidence in the combined LETAIRIS treatment group and more frequent than in the placebo group, with a difference of ≥1% between the LETAIRIS and placebo groups. |
|||
| Peripheral edema | 14 (11) | 45 (17) | 6 |
| Nasal congestion | 2 (2) | 15 (6) | 4 |
| Sinusitis | 0 (0) | 8 (3) | 3 |
| Flushing | 1 (1) | 10 (4) | 3 |
| Palpitations | 3 (2) | 12 (5) | 3 |
| Nasopharyngitis | 1 (1) | 9 (3) | 2 |
| Abdominal pain | 1 (1) | 8 (3) | 2 |
| Constipation | 2 (2) | 10 (4) | 2 |
| Dyspnea | 4 (3) | 11 (4) | 1 |
| Headache | 18 (14) | 38 (15) | 1 |
Most adverse drug reactions were mild to moderate and only nasal congestion was dose-dependent. Fewer patients receiving LETAIRIS had adverse events related to liver function tests compared to placebo.
Few notable differences in the incidence of adverse drug reactions were observed for patients by age or sex. Peripheral edema was similar in younger patients (<65 years) receiving LETAIRIS (14%; 29/205) or placebo (13%; 13/104), and was greater in elderly patients (≥65 years) receiving LETAIRIS (29%; 16/56) compared to placebo (4%; 1/28). The results of such subgroup analyses must be interpreted cautiously.
The incidence of treatment discontinuations due to adverse events other than those related to pulmonary hypertension during the clinical trials in patients with pulmonary arterial hypertension was similar for LETAIRIS (2%; 5/261 patients) and placebo (2%; 3/132 patients). The incidence of patients with serious adverse events other than those related to pulmonary hypertension during the clinical trials in patients with pulmonary arterial hypertension was similar for placebo (7%; 9/132 patients) and for LETAIRIS (5%; 13/261 patients).
6.2 Postmarketing Experience
The following adverse reaction was identified during postapproval use of LETAIRIS: Fluid retention [see Warnings and Precautions ( 5.3)].
Because this reaction was reported voluntarily from a population of uncertain size, it is not possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Studies with human liver tissue indicate that ambrisentan is metabolized by CYP3A4, CYP2C19, and uridine 5'-diphosphate glucuronosyltransferases (UGTs) 1A9S, 2B7S, and 1A3S. In vitro studies suggest that ambrisentan is a substrate of Organic Anion Transport Protein (OATP). In vitro studies show ambrisentan is a substrate but not an inhibitor of P-gp.
The drug interaction potential of ambrisentan is not well characterized because in vivo drug interaction studies were not conducted with the following types of drugs: strong inhibitors of CYP3A4 (atanazavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin), and CYP2C19 (omeprazole), strong inducers of CYP3A and 2C19 (rifampin), strong inhibitors of the transporters P-gp (cyclosporine A) and OATP (cyclosporine A, rifampin); and inducers of CYPs, UGTs and P-gp (rifampin). The impact of co-administration of such drugs on ambrisentan exposure is therefore unknown.
7.1 Cyclosporine A
Use caution when LETAIRIS is co-administered with cyclosporine A (see Warnings and Precautions 5.4).
7.2 Strong CYP3A or 2C19 Inhibitors
Use caution when LETAIRIS is co-administered with strong CYP3A-inhibitors (e.g., ketoconazole) or CYP2C19-inhibitors (e.g., omeprazole) [see Warnings and Precautions ( 5.5)].
7.3 Inducers of P-gp, CYPs, and UGTs
Use caution when LETAIRIS is co-administered with inducers of P-gp, CYPs, and UGTs.
7.4 Warfarin
In healthy volunteers receiving warfarin, daily doses of LETAIRIS (10 mg once daily) did not have a clinically significant effect on prothrombin time (PT), International Normalized Ratio (INR), or the pharmacokinetics of S-warfarin (CYP2C9 substrate) or R-warfarin (CYP3A4 substrate).
In patients with PAH receiving warfarin-type anticoagulants, concomitant administration of LETAIRIS did not result in a clinically relevant change in PT, INR or anticoagulant dose. Therefore, no dose-adjustments for warfarin or LETAIRIS are required when co-administered.
7.5 Sildenafil
In healthy volunteers receiving a single dose of sildenafil (20 mg), daily doses of LETAIRIS (10 mg once daily) did not have a clinically relevant effect on the pharmacokinetics of sildenafil or the active metabolite, n-desmethyl sildenafil. Similarly, daily doses of sildenafil (20 mg tid) did not have a clinically relevant effect on the pharmacokinetics of a single dose of LETAIRIS (10 mg). Therefore, no dose-adjustments for sildenafil or LETAIRIS are required when co-administered.
8 Use in Specific Populations
8.1 Pregnancy
Pregnancy Category X [see Contraindications ( 4.1)].
8.3 Nursing Mothers
It is not known whether ambrisentan is excreted in human milk. Breastfeeding while receiving LETAIRIS is not recommended. A preclinical study in rats has shown decreased survival of newborn pups (mid and high doses) and effects on testicle size and fertility of pups (high dose) following maternal treatment with ambrisentan from late gestation through weaning. Doses tested were 17x, 51x, and 170x (low, mid, high dose, respectively) the maximum oral human dose of 10 mg on a mg/mm2 basis.
8.4 Pediatric Use
Safety and effectiveness of LETAIRIS in pediatric patients have not been established.
8.5 Geriatric Use
In the two placebo-controlled clinical studies of LETAIRIS, 21% of patients were ≥65 years old and 5% were ≥75 years old. The elderly (age ≥65 years) showed less improvement in walk distances with LETAIRIS than younger patients did, but the results of such subgroup analyses must be interpreted cautiously. Peripheral edema was more common in the elderly than in younger patients.
8.6 Renal Impairment
The impact of renal impairment on the pharmacokinetics of ambrisentan has been examined using a population pharmacokinetic approach in PAH patients with creatinine clearances ranging between 20 and 150 mL/min. There was no significant impact of mild or moderate renal impairment on exposure to ambrisentan [see Clinical Pharmacology ( 12.3)]. Dose adjustment of LETAIRIS in patients with mild or moderate renal impairment is therefore not required. There is no information on the exposure to ambrisentan in patients with severe renal impairment.
The impact of hemodialysis on the disposition of ambrisentan has not been investigated.
8.7 Hepatic Impairment
The influence of pre-existing hepatic impairment on the pharmacokinetics of ambrisentan has not been evaluated. Because there is in vitro and in vivo evidence of significant metabolic and biliary contribution to the elimination of ambrisentan, hepatic impairment would be expected to have significant effects on the pharmacokinetics of ambrisentan [see Clinical Pharmacology ( 12.3)]. LETAIRIS is not recommended in patients with moderate or severe hepatic impairment. Use caution when administering LETAIRIS to patients with mild pre-existing impaired liver function who may require reduced doses of LETAIRIS [see Dosage and Administration ( 2.3)].
10 OVERDOSAGE
There is no experience with overdosage of LETAIRIS. The highest single dose of LETAIRIS administered to healthy volunteers was 100 mg and the highest daily dose administered to patients with PAH was 10 mg once daily. Massive overdosage could potentially result in hypotension that may require intervention.
11 DESCRIPTION
LETAIRIS is the brand name for ambrisentan, an endothelin receptor antagonist that is selective for the endothelin type-A (ETA) receptor. The chemical name of ambrisentan is (+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid. It has a molecular formula of C22H22N2O4 and a molecular weight of 378.42. It contains a single chiral center determined to be the (S) configuration and has the following structural formula:
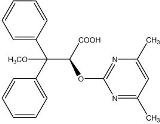
Figure 1 Ambrisentan Structural Formula
Ambrisentan is a white to off-white, crystalline solid. It is a carboxylic acid with a pKa of 4.0. Ambrisentan is practically insoluble in water and in aqueous solutions at low pH. Solubility increases in aqueous solutions at higher pH. In the solid state ambrisentan is very stable, is not hygroscopic, and is not light sensitive.
LETAIRIS is available as 5 mg and 10 mg film-coated tablets for once-daily oral administration. The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The tablets are film-coated with a coating material containing FD&C Red #40 aluminum lake, lecithin, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Each square, pale pink LETAIRIS tablet contains 5 mg of ambrisentan. Each oval, deep pink LETAIRIS tablet contains 10 mg of ambrisentan. LETAIRIS tablets are unscored.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endothelin-1 (ET-1) is a potent autocrine and paracrine peptide. Two receptor subtypes, ETA and ETB, mediate the effects of ET-1 in the vascular smooth muscle and endothelium. The primary actions of ETA are vasoconstriction and cell proliferation, while the predominant actions of ETB are vasodilation, antiproliferation, and ET-1 clearance.
In patients with PAH, plasma ET-1 concentrations are increased as much as 10-fold and correlate with increased mean right atrial pressure and disease severity. ET-1 and ET-1 mRNA concentrations are increased as much as 9-fold in the lung tissue of patients with PAH, primarily in the endothelium of pulmonary arteries. These findings suggest that ET-1 may play a critical role in the pathogenesis and progression of PAH.
Ambrisentan is a high affinity (Ki=0.011 nM) ETA receptor antagonist with a high selectivity for the ETA versus ETB receptor (>4000-fold). The clinical impact of high selectivity for ETA is not known.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a randomized, positive- and placebo-controlled, parallel-group study, healthy subjects received either LETAIRIS 10 mg daily followed by a single dose of 40 mg, placebo followed by a single dose of moxifloxacin 400 mg, or placebo alone. LETAIRIS 10 mg daily had no significant effect on the QTc interval. The 40 mg dose of LETAIRIS increased mean QTc at tmax by 5 ms with an upper 95% confidence limit of 9 ms. For patients receiving LETAIRIS 5-10 mg daily and not taking metabolic inhibitors, no significant QT prolongation is expected.
12.3 Pharmacokinetics
The absolute bioavailability of ambrisentan is not known. Ambrisentan is rapidly absorbed with peak concentrations occurring approximately 2 hours after oral administration in healthy subjects and PAH patients. Food does not affect its bioavailability. In vitro studies indicate that ambrisentan is a substrate of P-gp. Ambrisentan is highly bound to plasma proteins (99%). The elimination of ambrisentan is predominantly by non-renal pathways, but the relative contributions of metabolism and biliary elimination have not been well characterized. Based on in vitro data, interactions with strong inhibitors of P glycoprotein (P-gp), the Organic Anion Transport Protein (OATP), CYP3A4, CYP2C19, and uridine 5' diphosphate glucuronosyltransferases (UGTs) are possible [see Drug Interactions ( 7)]. The mean oral clearance of ambrisentan is 38 mL/min and 19 mL/min in healthy subjects and in PAH patients, respectively. Although ambrisentan has a 15-hour terminal half-life, the mean trough concentration of ambrisentan at steady-state is about 15% of the mean peak concentration and the accumulation factor is about 1.2 after long-term daily dosing, indicating that the effective half-life of ambrisentan is about 9 hours.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies of up to two years duration were conducted at starting doses of 10, 30, and 60 mg/kg/day in rats (8 to 48 times the maximum recommended human dose [MRHD] on a mg/m2 basis) and at 50, 150 and 250 mg/kg/day in mice (28 to 140 times the MRHD). In the rat study, the high and mid-dose male and female groups had their doses lowered to 40 and 20 mg/kg/day, respectively, in week 51 because of effects on survival. The high dose males and females were taken off drug completely in weeks 69 and 93, respectively. The only evidence of ambrisentan-related carcinogenicity was a positive trend in male rats, for the combined incidence of benign basal cell tumor and basal cell carcinoma of skin/subcutis in the mid-dose group (high-dose group excluded from analysis), and the occurrence of mammary fibroadenomas in males in the high-dose group. In the mouse study, high dose male and female groups had their doses lowered to 150 mg/kg/day in week 39 and were taken off drug completely in week 96 (males) or week 76 (females). In mice, ambrisentan was not associated with excess tumors in any dosed group.
Positive findings of clastogenicity were detected, at drug concentrations producing moderate to high toxicity, in the chromosome aberration assay in cultured human lymphocytes. There was no evidence for genetic toxicity of ambrisentan when tested in vitro in bacteria (Ames test) or in vivo in rats (micronucleus assay, unscheduled DNA synthesis assay).
The development of testicular tubular atrophy and impaired fertility has been linked to the chronic administration of endothelin receptor antagonists in rodents. Testicular tubular degeneration was observed in rats treated with ambrisentan for two years at doses ≥10 mg/kg/day (8-fold MRHD). Increased incidences of testicular findings were also observed in mice treated for two years at doses ≥50 mg/kg/day (28-fold MRHD). Effects on sperm count, sperm morphology, mating performance and fertility were observed in fertility studies in which male rats were treated with ambrisentan at oral doses of 300 mg/kg/day (236-fold MRHD). At doses of ≥10 mg/kg/day, observations of testicular histopathology in the absence of fertility and sperm effects were also present. There are insufficient data on the effects of ambrisentan or other endothelin receptor antagonists on testicular function in man.
14 Clinical Studies
14.1 Pulmonary Arterial Hypertension (PAH)
Two 12-week, randomized, double-blind, placebo-controlled, multicenter studies were conducted in 393 patients with PAH (WHO Group 1). The two studies were identical in design except for the doses of LETAIRIS and the geographic region of the investigational sites. ARIES-1 compared once-daily doses of 5 mg and 10 mg LETAIRIS to placebo, while ARIES-2 compared once-daily doses of 2.5 mg and 5 mg LETAIRIS to placebo. In both studies, LETAIRIS or placebo was added to current therapy, which could have included a combination of anticoagulants, diuretics, calcium channel blockers, or digoxin, but not epoprostenol, treprostinil, iloprost, bosentan, or sildenafil. The primary study endpoint was 6-minute walk distance. In addition, clinical worsening, WHO functional class, dyspnea, and SF-36® Health Survey were assessed.
Patients had idiopathic PAH (64%) or PAH associated with connective tissue disease (32%), HIV infection (3%), or anorexigen use (1%). There were no patients with PAH associated with congenital heart disease.
Patients had WHO functional class I (2%), II (38%), III (55%), or IV (5%) symptoms at baseline. The mean age of patients was 50 years, 79% of patients were female, and 77% were Caucasian.
Submaximal Exercise Capacity
Results of the 6-minute walk distance at 12 weeks for the ARIES-1 and ARIES-2 studies are shown in Table 2 and Figure 2.
|
Mean ± standard deviation |
||||||
|
† p-values are Wilcoxon rank sum test comparisons of LETAIRIS to placebo at Week 12 stratified by idiopathic PAH and non-idiopathic PAH patients |
||||||
| ARIES-1 | ARIES-2 | |||||
| Placebo (N=67) | 5 mg (N=67) | 10 mg (N=67) | Placebo (N=65) | 2.5 mg (N=64) | 5 mg (N=63) |
|
| Baseline | 342 ± 73 | 340± 77 | 342 ± 78 | 343 ± 86 | 347± 84 | 355 ± 84 |
| Mean change from baseline | -8 ± 79 | 23 ± 83 | 44 ± 63 | -10 ± 94 | 22 ± 83 | 49 ± 75 |
| Placebo-adjusted mean change from baseline | 31 | 51 | 32 | 59 | ||
| Placebo-adjusted median change from baseline | 27 | 39 | 30 | 45 | ||
| p-value† | 0.008 | <0.001 | 0.022 | <0.001 | ||
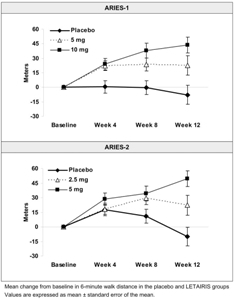
Figure 2 Mean Change in 6-minute Walk Distance
In both studies, treatment with LETAIRIS resulted in a significant improvement in 6-minute walk distance for each dose of LETAIRIS and the improvements increased with dose. An increase in 6-minute walk distance was observed after 4 weeks of treatment with LETAIRIS, with a dose-response observed after 12 weeks of treatment. Improvements in walk distance with LETAIRIS were smaller for elderly patients (age ≥65) than younger patients and for patients with secondary PAH than for patients with idiopathic PAH. The results of such subgroup analyses must be interpreted cautiously.
The effects of LETAIRIS on walk distances at trough drug levels are not known. Because only once daily dosing was studied in the clinical trials, the efficacy and safety of more frequent dosing regimens for LETAIRIS are not known. If exercise capacity is not sustained throughout the day in a patient, consider other PAH treatments that have been studied with more frequent dosing regimens.
Clinical Worsening
Time to clinical worsening of PAH was defined as the first occurrence of death, lung transplantation, hospitalization for PAH, atrial septostomy, study withdrawal due to the addition of other PAH therapeutic agents or study withdrawal due to early escape. Early escape was defined as meeting two or more of the following criteria: a 20% decrease in the 6-minute walk distance; an increase in WHO functional class; worsening right ventricular failure; rapidly progressing cardiogenic, hepatic, or renal failure; or refractory systolic hypotension. The clinical worsening events during the 12-week treatment period of the LETAIRIS clinical trials are shown in Table 3 and Figure 3.
|
Intention-to-treat population |
||||
|
Note: Patients may have had more than one reason for clinical worsening. |
||||
|
Nominal p-values |
||||
| ARIES-1 | ARIES-2 | |||
| Placebo
(N=67) | LETAIRIS
(N=134) | Placebo
(N=65) | LETAIRIS
(N=127) |
|
| Clinical worsening, no. (%) | 7 (10%) | 4 (3%) | 13 (22%) | 8 (6%) |
| Hazard ratio | 0.28 | 0.30 | ||
| p-value, Fisher exact test | 0.044 | 0.006 | ||
| p-value, Log-rank test | 0.030 | 0.005 | ||
There was a significant delay in the time to clinical worsening for patients receiving LETAIRIS compared to placebo. Results in subgroups such as the elderly were also favorable.
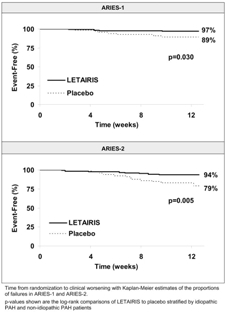
Figure 3 Time to Clinical Worsening
14.2 Long-Term Treatment of PAH
The long-term follow-up of the patients who were treated with LETAIRIS in the two pivotal studies and their open-label extension (N=383) shows that 95% were still alive at one year and 94% were still receiving LETAIRIS monotherapy. These uncontrolled observations do not allow comparison with a group not given LETAIRIS and cannot be used to determine the long-term effect of LETAIRIS.
14.3 Use in Patients with Prior Endothelin Receptor Antagonist (ERA) Related Liver Function Abnormalities
In an uncontrolled, open-label study, 36 patients who had previously discontinued endothelin receptor antagonists (ERAs: bosentan, an investigational drug, or both) due to aminotransferase elevations >3 x upper limit of normal (ULN) were treated with LETAIRIS. Prior elevations were predominantly moderate, with 64% of the ALT elevations <5 x ULN, but 9 patients had elevations >8 x ULN. Eight patients had been re-challenged with bosentan and/or the investigational ERA and all eight had a recurrence of aminotransferase abnormalities that required discontinuation of ERA therapy. All patients had to have normal aminotransferase levels on entry to this study. Twenty-five of the 36 patients were also receiving prostanoid and/or phosphodiesterase type 5 (PDE5) inhibitor therapy. Two patients discontinued early (including one of the patients with a prior 8 x ULN elevation). Of the remaining 34 patients, one patient experienced a mild aminotransferase elevation at 12 weeks on LETAIRIS 5 mg that resolved with decreasing the dosage to 2.5 mg, and that did not recur with later escalations to 10 mg. With a median follow-up of 13 months and with 50% of patients increasing the dose of LETAIRIS to 10 mg, no patients were discontinued for aminotransferase elevations. While the uncontrolled study design does not provide information about what would have occurred with re-administration of previously used ERAs or show that LETAIRIS led to fewer aminotransferase elevations than would have been seen with those drugs, the study indicates that LETAIRIS may be tried in patients who have experienced asymptomatic aminotransferase elevations on other ERAs after aminotransferase levels have returned to normal.
16 HOW SUPPLIED/STORAGE AND HANDLING
Because of the risk of liver injury and birth defects, LETAIRIS may be prescribed only through the LETAIRIS Education and Access Program (LEAP) by calling
1-866-664-LEAP (5327) or by logging on to www.letairis.com. Adverse events can also be reported directly via this number.
LETAIRIS film-coated, unscored tablets are supplied as follows:
| Package Configuration | Tablet Strength | NDC No. | Description of Tablet; Debossed on Tablet; Size |
| 30 count blister | 5 mg | 61958-0801-2 | Square convex; pale pink; “5” on side 1 and “GSI” on side 2; 6.6 mm Square |
| 30 count blister | 10 mg | 61958-0802-2 | Oval convex; deep pink; “10” on side 1 and “GSI” on side 2; 9.8 mm x 4.9 mm Oval |
Rx only
Store at 25 °C (77 °F); excursions permitted to 15-30 °C (59-86 °F) [see USP controlled room temperature]. Store LETAIRIS in its original packaging.
17 PATIENT COUNSELING INFORMATION
As a part of patient counseling, doctors must review the LETAIRIS Medication Guide with every patient [see FDA-Approved Medication Guide ( 17.5)].
17.1 Importance of Preventing Pregnancy
Patients should be advised that LETAIRIS may cause fetal harm. LETAIRIS treatment should only be initiated in women of childbearing potential following a negative pregnancy test. Women of childbearing potential should be informed of the importance of monthly pregnancy tests and the need to use two different forms of contraception including at least one primary form simultaneously during LETAIRIS treatment and for one month following treatment discontinuation. Primary forms of contraception other than tubal sterilization include hormonal (combination oral contraceptives, transdermal patch, injectables, implantables, or vaginal ring), IUD, and a partner's vasectomy. A Copper T 380A IUD or LNg 20 IUD can be used alone, i.e. without a secondary form of contraception, as can tubal sterilization. Patients should be instructed to immediately contact their physician if they suspect they may be pregnant [see Prescribing and Distribution Program for LETAIRIS ( 5.5)].
17.2 Adverse Liver Effects
Patients should be advised of the importance of monthly liver function testing and instructed to immediately report any symptoms of potential liver injury (such as anorexia, nausea, vomiting, fever, malaise, fatigue, right upper quadrant abdominal discomfort, jaundice, dark urine or itching) to their physician.
17.3 Hematological Change
Patients should be advised of the importance of hemoglobin testing.
17.4 Administration
Patients should be advised not to split, crush, or chew tablets.
17.5 FDA-Approved Medication Guide
*Sections or subsections omitted from the full prescribing information are not listed.
Gilead Sciences, Inc., Foster City, CA 94404
February 2008
LETAIRIS and the Gilead logo are trademarks of Gilead Sciences, Inc. Other brands noted herein are the property of their respective owners.
©2008 Gilead Sciences, Inc.
GS22-081-001
Medication Guide
LETAIRIS™ (le-TAIR-is)
Tablets
(ambrisentan)
Read this Medication Guide before you start taking LETAIRIS and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about LETAIRIS?
- Possible liver injury.
- LETAIRIS can cause liver injury. You must have a blood test to check your liver function before you start LETAIRIS and each month after that. Your doctor will order these blood tests. (See “ What are the possible side effects of LETAIRIS?” for information about the signs of liver problems.) Tell your doctor if you have had moderate or severe liver problems, including liver problems while taking other medicines.
- Serious birth defects.
- LETAIRIS can cause serious birth defects if taken during pregnancy. Women must not be pregnant when they start taking LETAIRIS or become pregnant during treatment. Women who are able to get pregnant must have a negative pregnancy test before beginning treatment with LETAIRIS and each month during treatment. Your doctor will decide when to do the test, depending on your menstrual cycle.
- Women who are able to get pregnant must use two different reliable forms of birth control at the same time, during LETAIRIS treatment and for one month after stopping LETAIRIS. Talk with your doctor or gynecologist (a doctor who specializes in female reproduction) to find out about how to prevent pregnancy. Do not have unprotected sex. Tell your doctor right away if you miss a menstrual period or think you may be pregnant.
LETAIRIS is available only through a restricted program called the LETAIRIS Education and Access Program (LEAP). To receive LETAIRIS, you must talk to your doctor, understand the benefits and risks of LETAIRIS, and agree to all of the instructions in the LEAP program.
What is LETAIRIS?
LETAIRIS is a prescription medicine to treat pulmonary arterial hypertension (PAH), which is high blood pressure in the arteries of your lungs.
LETAIRIS can improve your ability to exercise and it can help slow down the worsening of your physical condition and symptoms.
Who should not take LETAIRIS?
Do not take LETAIRIS if:
- you are pregnant, plan to become pregnant, or become pregnant during treatment with LETAIRIS. LETAIRIS can cause serious birth defects. (See “ What is the most important information I should know about LETAIRIS?”) Serious birth defects from LETAIRIS happen early in pregnancy.
- your blood tests show possible liver injury.
Tell your doctor about all your medical conditions and all the medicines you take including prescription and nonprescription medicines. LETAIRIS and other medicines may affect each other causing side effects. Do not start any new medicines until you check with your doctor.
LETAIRIS has not been studied in children.
How should I take LETAIRIS?
LETAIRIS will be mailed to you by a specialty pharmacy. Your doctor will give you complete details.
- Take LETAIRIS exactly as your doctor tells you. Do not stop taking LETAIRIS unless your doctor tells you.
- You can take LETAIRIS with or without food.
- Do not split, crush or chew LETAIRIS tablets.
- It will be easier to remember to take LETAIRIS if you take it at the same time each day.
- If you take more than your regular dose of LETAIRIS, call your doctor right away.
- If you miss a dose, take it as soon as you remember that day. Take your next dose at the regular time. Do not take two doses at the same time to make up for a missed dose.
- During treatment your doctor will test your blood for signs of side effects to your liver and red blood cells.
What should I avoid while taking LETAIRIS?
- Do not get pregnant while taking LETAIRIS. (See the serious birth defects section of “ What is the most important information I should know about LETAIRIS?”) If you miss a menstrual period, or think you might be pregnant, call your doctor right away.
- Breastfeeding is not recommended while taking LETAIRIS. It is not known if LETAIRIS can pass through your milk and harm your baby.
What are the possible side effects of LETAIRIS?
Serious side effects of LETAIRIS include:
- Possible liver injury. (See “ What is the most important information I should know about LETAIRIS?”) Call your doctor right away if you have any of these symptoms of liver problems: loss of appetite, nausea, vomiting, fever, unusual tiredness, right upper stomach pain, yellowing of the skin or the whites of your eyes (jaundice), dark urine, or itching.
- Serious birth defects. (See “ What is the most important information I should know about LETAIRIS?”)
- Low sperm count. LETAIRIS can lower sperm count in animals. If this happens in men, they may lose the ability to father children. Talk with your doctor if you have any questions or concerns.
The most common side effects of LETAIRIS are:
- Lowering of red blood cell count
- Swelling of hands, legs, ankles and feet (peripheral edema) and swelling all over the body (fluid retention)
- Stuffy nose (nasal congestion)
- Inflamed nasal passages (sinusitis)
- Hot flashes or getting red in the face (flushing)
- Feeling your heart beat (palpitations)
- Red and sore throat and nose
- Stomach pain
- Constipation
- Shortness of breath
- Headache
How should I store LETAIRIS?
Store LETAIRIS at less than 86 °F (30 °C), in the package it comes in.
General information about LETAIRIS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you have any concerns or questions about LETAIRIS, ask your doctor or other healthcare provider. This Medication Guide is only a summary of some important information about LETAIRIS. Your doctor can give you information about LETAIRIS that was written for healthcare professionals. Do not use LETAIRIS for any condition other than that for which it was prescribed. Do not share LETAIRIS with other people. It may harm them.
Call 1-866-664-LEAP (5327) or visit www.letairis.com or www.gilead.com for more information.
What are the ingredients in LETAIRIS?
Active ingredient: ambrisentan
Inactive Ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The tablets are film-coated with a coating material containing FD&C Red #40 aluminum lake, lecithin, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
This medication guide has been approved by the U.S. Food and Drug Administration.
Gilead Sciences, Inc., Foster City, CA 94404
February 2008
LETAIRIS and the Gilead logo are trademarks of Gilead Sciences, Inc. Other brands noted herein are the property of their respective owners.
©2008 Gilead Sciences, Inc.
GS22-081-001
| LETAIRIS (ambrisentan) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LETAIRIS (ambrisentan) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
Revised: 04/2008Gilead Sciences, Inc.