BRAVELLE
-
urofollitropin
Ferring Pharmaceuticals Inc.
----------
DESCRIPTION
Bravelle® is a product containing a highly purified preparation of human follicle stimulating hormone (hFSH) extracted from the urine of postmenopausal women. Human FSH consists of two non-covalently linked glycoproteins designated as the α and β subunits. The α subunit has 92 amino acids of which two are modified by attachment of carbohydrates. The β subunit has 111 amino acids of which two are modified by attachment of carbohydrates.
Bravelle® is a sterile, lyophilized powder intended for subcutaneous (SC) or intramuscular (IM) injection after reconstitution with sterile 0.9% Sodium Chloride Injection, USP. Each vial of Bravelle® contains 82.5 International Units (IU) of Follicle Stimulating Hormone (FSH) activity, 23 mg Lactose Monohydrate, 0.005 mg Polysorbate 20, and Sodium Phosphate buffer (Sodium Phosphate dibasic, Heptahydrate and Phosphoric acid) for pH adjustments, which, when reconstituted with diluent, will deliver 75 IU of FSH. Bravelle® contains up to 2% luteinizing hormone (LH) activity based on bioassay. Human Chorionic Gonadotropin (hCG) is not detected in Bravelle®. When stored at 3° to 25°C, up to 40% of the α-subunits may be oxidized.
The in vivo biological activity of urofollitropin for injection, purified is determined by using reference standards calibrated against the First International Standard for follicle- stimulating hormone, (FSH, Urofollitropin), Urinary, Human for Bioassay, National Institute for Biological Standards and Control (NIBSC) at its 46th meeting in 1995.
FSH is a glycoprotein that is acidic and water-soluble. Therapeutic class: Infertility.
Therapeutic class: Infertility.
CLINICAL PHARMACOLOGY
Bravelle® administered for 7 to 12 days produces ovarian follicular growth in women who do not have primary ovarian failure. Treatment with Bravelle® in most instances results only in follicular growth and maturation. When sufficient follicular maturation has occurred, hCG must be given to induce ovulation.
PHARMACOKINETICS
Single doses of 225 IU and multiple daily doses (7 days) of 150 IU of Bravelle® were administered to healthy volunteer female subjects while their endogenous FSH was suppressed. Sixteen subjects received Bravelle® SC and 12 received the drug IM. Serum FSH concentrations were determined. Based on the steady state ratio of FSH Cmax and AUC, SC and IM administration of Bravelle® were not bioequivalent. Multiple doses of Bravelle® IM resulted in Cmax and AUC of 77.7% and 81.8% compared to multiple doses of Bravelle® SC. The FSH pharmacokinetic parameters for single and multiple dose Bravelle®, administered SC and IM are in Table 1.
| Single Dose (225 IU) | Multiple Dose X 7 (150 IU) | |||
| PK Parameters | SC | IM | SC | IM |
| Cmax (mIU/mL) | 6.0 (1.7) | 8.8 (4.5) | 14.8 (2.9) | 11.5 (2.9) |
| Tmax (hrs) | 20. (7.7) | 17.4 (12.2) | 9.6 (2.1) | 11.3 (8.4) |
| AUCobs (mlU•hr/mL) | 379 (111) | 331 (179) | 234.7 (77.0) | 192.1 (52.3) |
| T1/2 (hrs) | 31.8 | 37 | 20.6 | 15.2 |
| Ka (hr -1) | 0.0500 (0.0231) | 0.1408 (0.1227) | 0.0905 (0.0383) | 0.0358 (0.0108) |
Absorption
The maximum plasma concentration of FSH was attained at 20.5 and 17.4 hours following SC and IM single dose administration, respectively. However, following multiple dosing, it was attained at approximately 10 hours following both routes of administration.
Elimination
The mean elimination half-lives of FSH for SC and IM single dosing are 31.8 and 37 hours, respectively. However, following multiple dosing (X 7 days) they are 20.6 and 15.2 hours for SC and IM, respectively.
CLINICAL STUDIES
The efficacy of Bravelle® was established in two randomized, active controlled, multi-center studies, one for in-vitro fertilization [IVF] and one for ovulation induction [0I].
Ovulation Induction
In the randomized, controlled ovulation induction study, patients underwent pituitary suppression with a GnRH agonist before being randomized to Bravelle® SC, Bravelle® IM or a commercial recombinant FSH product administered SC. A total of 111 oligo-anovulatory patients were randomized of whom 72: received Bravelle®, starting at a dose of 150 IU daily for 5 days. This was followed by individual titration of the dose from 75 to 450 IU daily Based on ultrasound and estradiol (E2) levels. The total duration of dosing did not exceed 12 days. Results for the Intent-To-Treat Population are summarized in Table 2.
| Bravelle®SC | Bravelle® IM | |
| Parameter | N=35 | N=37 |
| Ovulation (%) | 24 (68.6) | 26 (70.3) |
| Received hCG (%) | 25 (71.4) | 28 (75.7) |
| Mean Peak Serum E2 (pg/mL) (SD) | 976.5 (680.6) | 893.2 (815.2) |
| Chemical Pregnancy (%) | 11 (31.4) | 8 (21.6) |
| Clinical Pregnancy (%) | 9 (25.7) | 7 (18.9) |
| Continuing Pregnancy (%) | 9 (25.7) | 7 (18.9) |
| Pts. w/Live Births (%) | 9 (25.7) | 6 (16.2) |
Assisted Reproductive Technologies [ART]
In the randomized, controlled IVF study FPI FSH 2001-01, patients underwent pituitary suppression with a GnRH agonist before being randomized to Bravelle® SC or a commercial recombinant FSH product administered SC. A total of 120 patients were randomized of whom 60 received Bravelle®, starting at a dose of 225 IU daily for 5 days. This was followed by individual titration of the dose from 75 to 450 IU daily based on ultrasound and estradiol (E2) levels. The total duration of dosing did not exceed 12 days. Results are summarized in Table 3 for the Intent-To-Treat population.
| Bravelle®SC | |
| Parameter | n=60 |
| Mean Total Oocytes Retrieved Per Patient (SD) | 11.8 (6.3) |
| Mean Mature Oocytes Retrieved Per Patient (SD) | 9.0 (5.7) |
| Patients with Oocyte Retrieval (%) | 57 (95.0) |
| Patients with Embryo Transfer (%) | 57 (95.0) |
| Patients with Chemical Pregnancy (%) | 28 (46.6) |
| Patients with Clinical Pregnancy (%) | 25 (41.7) |
| Patients with Continuing Pregnancy (%) | 23 (38.3) |
INDICATIONS AND USAGE
Ovulation Induction
Bravelle® administered SC or IM in conjunction with hCG, is indicated for ovulation induction in patients who have previously received pituitary suppression.
Multifollicular Development during ART
Bravelle® administered SC in conjunction with hCG is indicated for multiple follicular development (controlled ovarian stimulation) during ART cycles in patients who have previously received pituitary suppression.
Selection of Patients
- Before treatment with Bravelle® is instituted, a thorough gynecologic and endocrinologic evaluation must be performed. Except for those patients enrolled in an in vitro fertilization program, this should include a hysterosalpingography (to rule out uterine and tubal pathology) and documentation of anovulation by means of basal body temperature, serial vaginal smears, examination of cervical mucus, determination of serum (or urine) progesterone, urinary pregnanediol and endometrial biopsy. Patients with tubal pathology should receive Bravelle® only if enrolled in an in vitro fertilization program.
- Primary ovarian failure should be excluded by the determination of gonadotropin levels.
- Careful examination should be made to rule out the presence of an early pregnancy.
- Patients in late reproductive life have a greater predilection to endometrial carcinoma as well as a higher incidence of anovulatory disorders. Cervical dilation and curettage should always be done for diagnosis before starting Bravelle® therapy in such patients who demonstrate abnormal uterine bleeding or other signs of endometrial abnormalities.
- Evaluation of the husband's fertility potential should be included in the workup.
CONTRAINDICATIONS
Bravelle® is contraindicated in women who have:
- A high FSH level indicating primary ovarian failure.
- Uncontrolled thyroid and adrenal dysfunction.
- An organic intracranial lesion such as pituitary tumor.
- The presence of any cause of infertility other than anovulation.
- Abnormal bleeding of undetermined origin.
- Ovarian cysts or enlargement not due to polycystic ovary syndrome.
- Prior hypersensitivity to urofollitropins, purified.
- Bravelle® is contraindicated in women who are pregnant and may cause fetal harm when administered to a pregnant woman. There are limited human data on the effects of Bravelle® when administered during pregnancy.
WARNINGS
Bravelle® is a drug that should only be used by physicians who are thoroughly familiar with infertility problems. It is a potent gonadotropic substance capable of causing Ovarian Hyperstimulation Syndrome [OHSS] with or without pulmonary or vascular complications in women. Bravelle® therapy requires a certain time commitment by physicians and supportive health professionals, and its use requires the availability of appropriate monitoring facilities (see PRECAUTIONS - Laboratory Tests). Bravelle® should be used with a great deal of care.
Overstimulation of the Ovary During Bravelle® Therapy
Ovarian Enlargement: Mild to moderate uncomplicated ovarian enlargement which may be accompanied by abdominal distension and/or abdominal pain occurs in approximately 20% of those treated with follitropin and hCG, and generally regresses without treatment within two or three weeks.
In order to minimize the hazard associated with the occasional abnormal ovarian enlargement, which may occur with FSH - hCG therapy, the lowest dose consistent with expectation of good results should be used. Careful monitoring of ovarian response can further minimize the risk of overstimulation.
If the ovaries are abnormally enlarged on the last day of Bravelle® therapy, hCG should not be administered in the course of therapy; this will reduce the chances of development of the Ovarian Hyperstimulation Syndrome.
OHSS: OHSS is a medical event distinct from uncomplicated ovarian enlargement. OHSS may progress rapidly to become a serious medical event. It is characterized by an apparent dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. The following symptomatology has been seen with cases of OHSS: abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic events (see "Pulmonary and Vascular Complications" below). Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with the Ovarian Hyperstimulation Syndrome (OHSS).
In a clinical study of ovulation induction, 6 of 72 (8.33%) Bravelle® treated women developed OHSS and two were classified as severe. In a clinical study for multiple follicular development during IVF, 3 of 60 Bravelle® treated women developed OHSS and 1 was classified as severe. Cases of OHSS are more common, more severe and more protracted if pregnancy occurs. OHSS develops rapidly; therefore patients should be followed for at least two weeks after hCG administration. Most often, OHSS occurs after treatment has been discontinued and reaches its maximum at about 7 to 10 days after treatment. Usually, in cases where OHSS may be developing prior to hCG administration (see PRECAUTIONS - Laboratory Tests), the hCG should be withheld.
If severe OHSS occurs, treatment must be stopped and the patient should be hospitalized.
A physician experienced in the management of the syndrome, or who is experienced in the management of fluid and electrolyte imbalances should be consulted.
Pulmonary and Vascular Complications
Serious pulmonary conditions (e.g. atelectasis, acute respiratory distress syndrome) have been reported. In addition, thromboembolic events both in association with, and separate from, the Ovarian Hyperstimulation Syndrome have been reported following FSH therapy. Intravascular thrombosis and embolism, which may originate in venous or arterial vessels, can result in reduced blood flow to critical organs or the extremities. Sequelae of such events have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb. In rare cases, pulmonary complications and/or thromboembolic events have resulted in death.
Multiple Pregnancies
Multiple pregnancies have occurred following treatment with Bravelle® SC and IM.
Pregnancy outcomes in a controlled study of 72 patients undergoing ovulation induction with Bravelle® are shown in Table 4.
| Bravelle® SC | Bravelle® IM | |
| Parameter | N(%) | N(%) |
| Total Continuing Pregnancies | 9(100) | 7(100) |
| Singlets | 3(33.3) | 5(71.4) |
| Total No. with Multiple Pregnancies | 6(66.7) | 2(28.6) |
| Twins | 4 | 0 |
| Triplets | 2 | 0 |
| Quadruplets | 0 | 1 |
| Quintuplets | 0 | 0 |
| Sextuplets | 0 | 1 |
The pregnancy outcomes in a controlled study of 60 patients undergoing treatment with Bravelle® in IVF are shown in Table 5.
| Bravelle® SC | |
| Parameter (%) | N(%) |
| Total No. of Continuing Pregnancies | 23(100) |
| Singlets | 15 (65.2) |
| Total No. of Multiple Pregnancies | 8 (34.8) |
| Twins | 5 |
| Triplets | 3 |
The patient and her partner should be advised of the potential risk of multiple births before starting treatment.
Hypersensitivity/Anaphylactic Reactions
Hypersensitivity/anaphylactic reactions associated with urofollitropins for injection, purifi ed administration have been reported in some patients. These reactions presented as generalized urticaria, facial edema, angioneurotic edema, and/or dyspnea suggestive of laryngeal edema. The relationship of these symptoms to uncharacterized urinary proteins is uncertain.
PRECAUTIONS
General
Careful attention should be given to the diagnosis of infertility in the selection of candidates for Bravelle® therapy (see "INDICATIONS AND USAGE - Selection of Patients")
Information for Patients
Prior to therapy with Bravelle® patients should be informed of the duration of treatment and the monitoring of their condition that will be required. Possible adverse reactions (see ADVERSE REACTIONS section) and the risk of multiple births should also be discussed.
Laboratory Tests
The combination of both estradiol levels and ultrasonography are useful for monitoring the growth and development of follicles, timing hCG administration, as well as minimizing the risk of the Ovarian Hyperstimulation Syndrome and multiple gestations.
The clinical confirmation of ovulation, is determined by:
a. A rise in basal body temperature,
b. Increase in serum progesterone, and
c. Menstruation following the shift in basal body temperature.
When used in conjunction with indices of progesterone production, sonographic visualization of the ovaries will assist in determining if ovulation has occurred. Sonographic evidence of ovulation may include the following:
a. Fluid in the cul-de-sac,
b. Ovarian stigmata, and
c. Collapsed follicle.
Because of the subjectivity of the various tests for the determination of follicular maturation and ovulation, it cannot be overemphasized that the physician should choose tests with which he/she is thoroughly familiar.
Carcinogenesis and Mutagenesis
Long-term toxicity studies in animals and in vitro mutagenicity tests have not been performed to evaluate the carcinogenic potential of urofollitropin for injection, purified.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in the nursing infant from Bravelle®, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
The safety of Bravelle® was examined in four clinical studies that enrolled a total of 222 patients receiving Bravelle® including 72 for ovulation induction and 150 for IVF.
All adverse events (without regard to causality assessment) occurring ≥2% incidence in the clinical study patients receiving Bravelle® are listed in Table 6, (FPI FSH 99-03 study for ovulation induction) and Table 7 (FPI FSH 99-04, FPI FSH 99-05 and FPI FSH 2001-01 studies for IVF).
| All Patients with Adverse Events ≥ 2% | ||
| Adverse Events(%) | Bravelle® SC | Bravelle® IM |
| N=35 | N=37 | |
| Genitourinary/Reproductive | ||
| OHSS | 4 (11.4) | 2 (5.4) |
| Vaginal Hemorrhage | 3 (8.6) | 0 (0.0) |
| Ovarian Disorder (Pain, Cyst) | 1 (2.9) | 3 (8.1) |
| Urinary tract infection | 0 | 1 (2.7) |
| Cervix disorder | 1 (2.9) | 0 |
| Gastrointestinal | ||
| Nausea | 2 (5.7) | 0 (0.0) |
| Enlarged Abdomen | 1 (2.9) | 1 (2.7) |
| Abdominal Pain | 1 (2.9) | 2 (5.4) |
| Vomiting | 0 | 1 (2.7) |
| Constipation | 0 | 1 (2.7) |
| Diarrhea | 0 | 1 (2.7) |
| Metabolic/Nutritional | ||
| Dehydration | 0 | 1 (2.7) |
| Weight gain | 1 (2.9) | 0 |
| Skin/Appendages | ||
| Acne | 1 (2.9) | 0 |
| Exfoliative dermatitis | 0 | 1 (2.7) |
| Other Body Systems | ||
| Headache | 4 (11.4) | 3 (8.1) |
| Pain | 2 (5.7) | 0 (0.0) |
| Neck pain | 0 | 1 (2.7) |
| Respiratory Disorder | 2 (5.7) | 0 (0.0) |
| Hot Flashes | 2 (5.7) | 0 (0.0) |
| Fever | 0 | 1 (2.7) |
| Hypertension | 0 | 1 (2.7) |
| Emotional lability | 0 | 1 (2.7) |
| Depression | 0 | 1 (2.7) |
| Accidental injury | 0 | 1 (2.7) |
| All Patients with Adverse Events ≥ 2% | |
| Adverse Events (%) | Bravelle® SC N=150 |
| Genitourinary/Reproductive | |
| Vaginal hemorrhage | 7 (4.7) |
| Post retrieval pain | 12 (8.0) |
| Pelvic pain/cramps | 10 (6.7) |
| OHSS | 9 (6.0) |
| Uterine spasms | 4 (2.7) |
| Vaginal spotting | 4 (2.7) |
| Urinary tract infection | 5 (3.3) |
| Ovarian disorder | 3 (2.0) |
| Breast tenderness | 3 (2.0) |
| Vaginal Discharge | 4 (2.7) |
| Infection fungal | 3 (2.0) |
| Gastrointestinal | |
| Abdominal cramps | 21 (14.0) |
| Nausea | 13 (8.7) |
| Abdominal pain | 7 (4.7) |
| Abdominal fullness/enlargement | 10 (6.7) |
| Constipation | 3 (2.0) |
| Other Body Systems | |
| Headache | 19 (12.7) |
| Pain | 8 (5.3) |
| Rash | 4 (2.7) |
| Respiratory disorder | 6 (4.0) |
| Sinusitis | 3 (2.0) |
| Injection site reaction | 6 (4.0) |
| Hot flash | 6 (4.0) |
| Emotional lability | 3 (2.0) |
The following medical events have been reported subsequent to pregnancies resulting from gonadotropin therapy in published clinical studies:
- Spontaneous Abortion
- Ectopic Pregnancy
- Premature Labor
- Postpartum fever
- Congenital abnormalities
The following adverse reactions have been previously reported during urofollitropin for injection, purified therapy:
- Pulmonary and vascular complications (see WARNINGS)
- Adnexal torsion (as a complication of ovarian enlargement),
- Mild to moderate ovarian enlargement,
- Hemoperitoneum,
- There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have undergone multiple drug regimens for ovulation induction; however, a causal relationship has not been established.
OVERDOSAGE
Aside from possible ovarian hyperstimulation (see WARNINGS) and multiple gestations (see WARNINGS), little is known concerning the consequences of acute overdosage with Bravelle®.
DOSAGE AND ADMINISTRATION
Dosage:
Infertile patients with oligo-anovulation:
The dose of Bravelle® to stimulate development of ovarian follicles must be individualized for each patient. The lowest dose consistent with achieving good results based on clinical experience and reported clinical data should be used.
The recommended initial dose of Bravelle® for patients who have received GnRH agonist or antagonist pituitary suppression is 150 IU daily administered SC or IM for the first 5 days of treatment. Based on clinical monitoring (including serum estradiol levels and vaginal ultrasound results) subsequent dosing should be adjusted according to individual patient response. Adjustments in dose should not be made more frequently than once every 2 days and should not exceed more than 75 to 150 IU per adjustment. The maximum daily dose of Bravelle® should not exceed 450 IU and in most cases dosing beyond 12 days is not recommended.
If patient response to Bravelle® is appropriate, hCG (5000 to 10,000 USP units) should be given 1 day following the last dose of Bravelle®. The hCG should be withheld if the serum estradiol is greater than 2000 pg/mL, if the ovaries are abnormally enlarged or if abdominal pain occurs, and the patient should be advised to refrain from intercourse. These precautions may reduce the risk of Ovarian Hyperstimulation Syndrome and multiple gestations. Patients should be followed closely for at least 2 weeks after hCG administration. If there is inadequate follicle development or ovulation without subsequent pregnancy, the course of treatment with Bravelle® may be repeated. The couple should be encouraged to have intercourse daily, beginning on the day prior to the administration of hCG until ovulation becomes apparent from the indices employed for the determination of progestational, activity. In the light of the foregoing indices and parameters mentioned, it should become obvious that, unless a physician is willing to devote considerable time to these patients and be familiar with and conduct the necessary laboratory studies, he/she should not use Bravelle®.
Assisted Reproductive Technologies:
The recommended initial dose of Bravelle® for patients undergoing IVF and donor egg patients who have received GnRH agonist or antagonist pituitary suppression is 225 IU daily administered SC for the first 5 days of treatment. Based on clinical monitoring (including serum estradiol levels and vaginal ultrasound results) subsequent dosing should be adjusted according to individual patient response. Adjustments in dose should not be made more frequently than once every 2 days and should not exceed more than 75 to 150 IU per adjustment. The maximum daily dose of Bravelle® given should not exceed 450 IU and in most cases dosing beyond 12 days is not recommended.
Once adequate follicular development is evident, hCG (5,000-10,000 USP units) should be administered to induce final follicular maturation in preparation for oocyte retrieval. The administration of hCG must be withheld in cases where the ovaries are abnormally enlarged on the last day of therapy. This should reduce the chance of developing OHSS.
HOW SUPPLIED
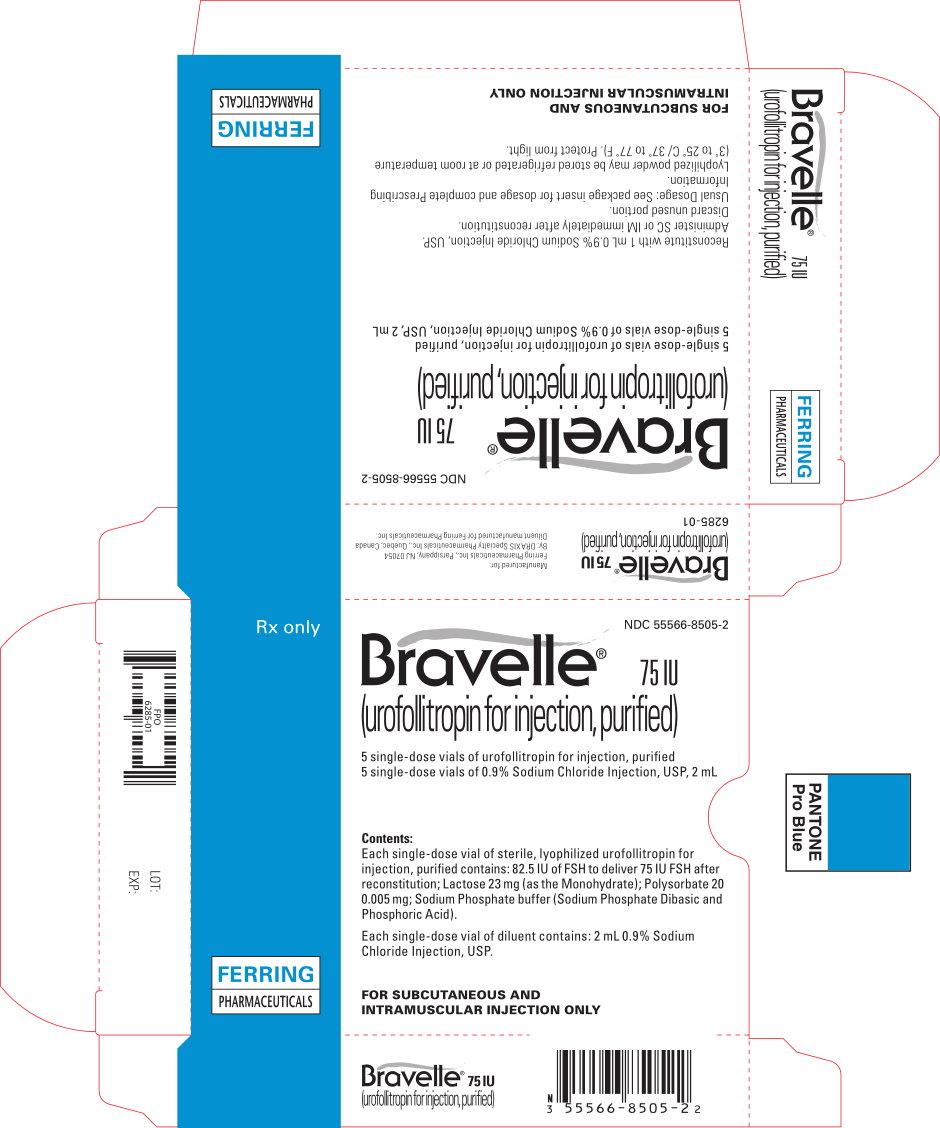
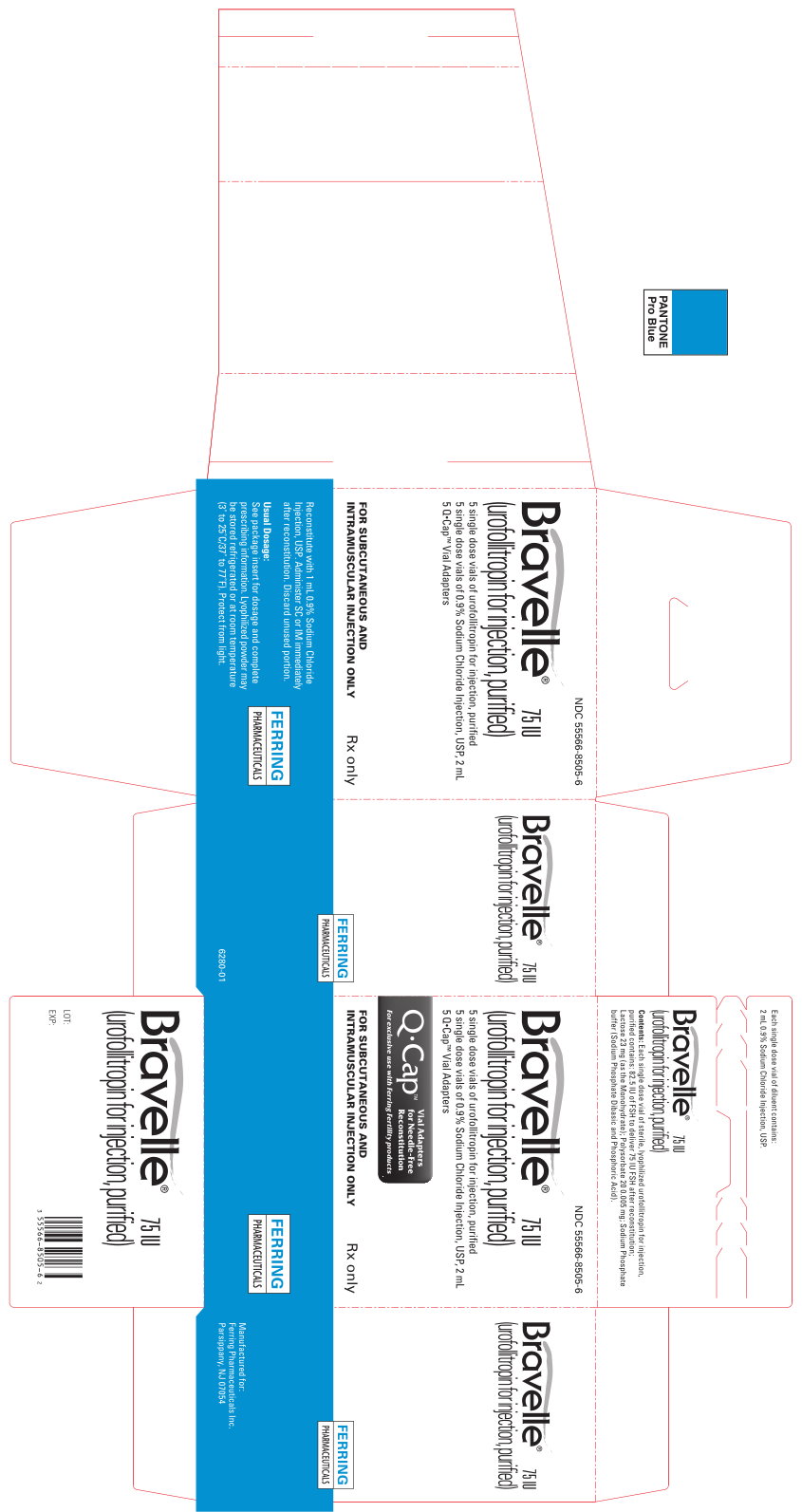
Bravelle® (urofollitropin for injection, purified) is supplied in a sterile, lyophilized, single dose vial containing 82.5 IU of FSH, to deliver 75 IU FSH after reconstituting with the diluent.
Each vial is available with an accompanying vial of sterile diluent containing 2 mL of 0.9% Sodium Chloride Injection, USP.
75 IU FSH activity, supplied as:
NDC 55566-8505-2: Box of 5 vials + 5 vials diluent.
NDC 55566-8505-6: Box of 5 vials + 5 vials diluent + 5 Q●Cap™ vial adaptors.
Lyophilized powder may be stored refrigerated or at room temperature (3° to 25° C/37° to 77°F). Protect from light. Use immediately after reconstitution. Discard unused material.
Rx only
Vials of sterile diluent of 0.9% Sodium Chloride Injection, USP, manufactured for Ferring Pharmaceuticals Inc.
Manufactured for:
FERRING PHARMACEUTICALS INC.
PARSIPPANY, NJ 07054
6317-01
09/08
PATIENT INFORMATION
BRAVELLE®
(urofollitropin for injection, purified)
Read the Patient Information that comes with BRAVELLE® before you start taking it and each time you get a refill, as there may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
What is BRAVELLE®?
BRAVELLE® is a medicine that can help women get pregnant. It is given by injection. You should use BRAVELLE® as your doctor indicates. BRAVELLE® contains a hormone that helps stimulate eggs to grow and mature.
BRAVELLE® is used for:
- Women whose ovaries are basically healthy but are unable to develop eggs and who do not become pregnant as a result. It is not for women who suffer from ovarian failure.
- Women who are going to have in vitro fertilization (IVF) and need to produce many eggs.
Before using BRAVELLE® you will have a health checkup. This will help your doctor learn more about your medical condition. If your ovaries need help stimulating eggs, your doctor may prescribe BRAVELLE®. You may also be asked to take other medicines. Many women take more than one medicine as part of a pregnancy plan.
Who should not use BRAVELLE®?
Do not use BRAVELLE® if you:
- Have ovaries that are not able to produce eggs even with help.
- Are pregnant or think you may be pregnant.
- Have problems with your thyroid gland or adrenal gland that are not controlled by medication.
- Have cancer in your female organs (ovaries breast, uterus).
- Have a tumor of your pituitary gland or other tumor in your brain.
- Have abnormal bleeding from your vagina.
- Have ovarian cysts or enlarged ovaries not due to a condition known as polycystic ovary syndrome (PCOS).
What should I tell my doctor before using BRAVELLE®?
Tell your doctor about any other medicines you are taking. This includes any prescription or nonprescription medicines, vitamins, or herbal supplements.
How should I use BRAVELLE®?
- Use BRAVELLE® just as your doctor says. You will be given the amount that is right for you. Do not change the amount unless your doctor tells you to. Your doctor will tell you how much BRAVELLE® to use each day and how many days to take it.
- If you use too much or too little BRAVELLE®, call your doctor right away.
- If you forget to use BRAVELLE®, call your doctor right away. Do not double the amount of BRAVELLE® that you are taking.
- Do not give BRAVELLE® to anyone else, including other women who are having trouble getting pregnant.
What are possible side effects with BRAVELLE®?
- BRAVELLE® sometimes excites the ovaries too much. This may cause pelvic pain or breathing problems. It may also make you urinate less. In rare cases, patients with this problem have had serious lung problems, including fl uid in the lungs, trouble breathing, and worsening of asthma.
- Blood clots and strokes
Call your doctor or get medical help right away if you have: - Severe pelvic pain, chest pain, or abdominal pain
- Nausea
- Vomiting
- Sudden weight gain
- Bloating
- Trouble breathing
BRAVELLE® may cause you to have twins or multiple births. Your doctor will talk to you about this.
The most common side effects with BRAVELLE® are headache, vaginal bleeding, nausea, and hot flashes. Sometimes there is a reaction at the spot where you give yourself the injection. This can include bruising, pain, or redness.
These are not all of the side effects of BRAVELLE®. As with any drug, tell your doctor about any side effects or other symptoms you have, or any physical changes.
Step-by-Step Instructions for Reconstitution and Subcutaneous Administration of BRAVELLE®
-
Before you start
- Wash your hands well with soap. Use a soap that kills bacteria. Dry your hands with a clean towel.
- Use alcohol to clean the surface where you will be working. Lay out all the things you will need.
- Have these supplies ready as you start to prepare BRAVELLE®:
- The vials of BRAVELLE® powder and sodium chloride 0.9% (sterile diluent)
- Alcohol pads, rubbing alcohol, and gauze wipes
- A sterile syringe and needle
- Q•Cap™ (for exclusive use with Ferring fertility products) that is packaged with your medicine
- A “Sharps container” or a large jar for getting rid of needles after you are done with them. The jar should be made from hard plastic or metal. Make sure it has a lid. You can also put used syringes and empty bottles of medicine in the jar.
-
Preparing your BRAVELLE® and filling the syringe
- Remove the syringe from the wrapper. If there is a capped needle on the syringe, remove the needle by twisting it counterclockwise. Open Q•Cap™ by peeling back the label and set the blister pouch with the Q•Cap™ aside. Do not take the Q•Cap™ out of the pouch at this time. Do not touch the ends of the Q•Cap™.
- Remove the plastic caps from the vials of BRAVELLE® powder and sterile diluent.
- Wipe the tops of the vials with alcohol and allow them to dry. Do not touch the tops of the vials after you have wiped them.
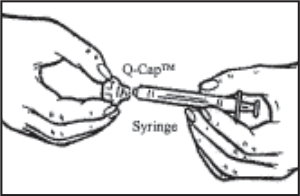
- Place the sterile diluent vial on the counter. Remove the Q•Cap™ from the blister pouch by grasping its side. Carefully twist the syringe onto the Luer end (connector) of the Q•Cap™ until you feel a slight resistance. Do not touch the spike at the end of the Q•Cap™.
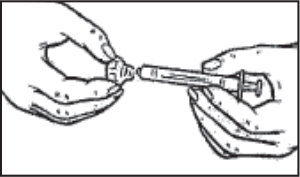
- Withdraw the syringe plunger to the volume of diluent that is to be removed from the vial. This is normally 1 mL, but be sure to follow your doctor’s instructions on the amount of diluent to use.
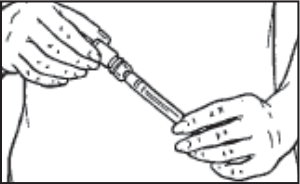
- Hold the syringe and place the spike end of the Q•Cap™ over the top of the sterile diluent vial. Push the tip of the Q•Cap™ into the rubber stopper of the vial until you feel a slight resistance. Be careful not to push down on on the syringe plunger during this step.
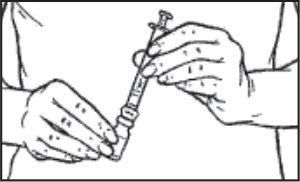
- Push the syringe plunger down to transfer the air from the syringe into the vial. Keeping the syringe and Q•Cap™ together, turn the vial upside down and pull back on the syringe plunger to withdraw the desired amount of sterile diluent from the vial, as directed by your doct
- Place the vial on the counter. Remove the Q•Cap™ and syringe from the vial by pulling up on the syringe barrel. Discard the diluent vial
- Hold the BRAVELLE® (urofollitropin for injection, purifi ed) vial in one hand. Grasp the sides of the syringe with your other hand and pace the tip of the Q•Cap™ over the top of the vial. Push the tip of the Q•Cap™ into the rubber stopper of the vial until you feel a slight resistance. Be very careful not to push down on the syringe plunger during this step.
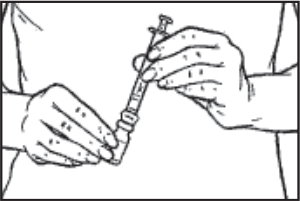
- Slowly inject the sterile diluent into the vial containing the BRAVELLE® powder. The BRAVELLE® powder will dissolve quickly. Mix by gently swirling until the powder is completely dissolved. Do not shake the vial because this will create bubbles.
- As soon as the powder has completely dissolved, turn the vial upside down and withdraw all of the BRAVELLE® into the syringe.
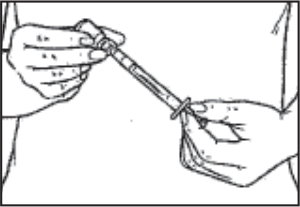
Note to patients who need multiple vials: Some patients need to take more than 1 vial of BRAVELLE®. If your doctor tells you to do this, prepare the fi rst vial of BRAVELLE® with sterile diluent. (Repeat Step 2.) Then use the liquid in the syringe to mix up to 5 more vials of medicine. Your doctor will tell you how many vials of BRAVELLE® to use.
- Remove the syringe from the wrapper. If there is a capped needle on the syringe, remove the needle by twisting it counterclockwise. Open Q•Cap™ by peeling back the label and set the blister pouch with the Q•Cap™ aside. Do not take the Q•Cap™ out of the pouch at this time. Do not touch the ends of the Q•Cap™.
-
Removing the Q•Cap™ and adding the subcutaneous needle
- When you have finished reconstituting the last vial necessary for your injection and have withdrawn all the medication into the syringe, twist the syringe counterclockwise while holding the Q•Cap™ steady to remove the syringe from the Q•Cap™. Discard the Q•Cap™ with the attached medication vial.
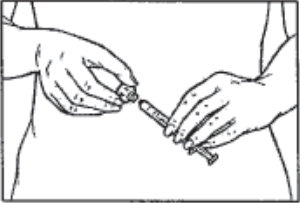
- You are now ready to attach the injection needle to the syringe for your injection.
Note: Please follow your doctor’s instructions on which needle to use. - While holding the syringe pointing upward, twist the needle clockwise onto the syringe.
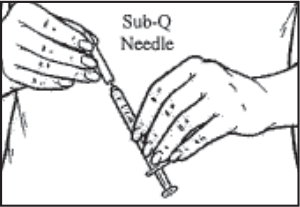
- Hold the syringe straight up. Pull back slightly on the plunger and tap the syringe so that any air bubbles rise to the top. Slowly press the plunger until all the air is out of the syringe and a small drop of solution forms at the tip of the needle.
- Tap the syringe to remove the drop of solution at the tip of the needle.
- Carefully recap the needle to keep it sterile. The solution is now ready for injection.
- When you have finished reconstituting the last vial necessary for your injection and have withdrawn all the medication into the syringe, twist the syringe counterclockwise while holding the Q•Cap™ steady to remove the syringe from the Q•Cap™. Discard the Q•Cap™ with the attached medication vial.
-
Injecting BRAVELLE®
- You should inject BRAVELLE® into the skin on your abdomen a few inches below your navel, to the left or right. Each day, inject at a different spot to help reduce soreness. For example, on day 1, inject yourself at spot 1. The next day, use spot 2. And so on. On the fifth day, begin again with spot 1.
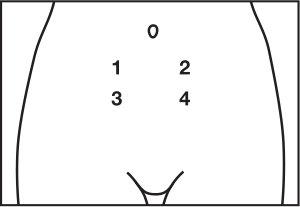
- Carefully clean the injection spot with an alcohol pad. Allow the spot to air-dry.
- Remove the needle cap from the syringe.
- Hold the syringe in one hand. Use your other hand to gently grasp the skin in the injection site area between your thumb and index finger.
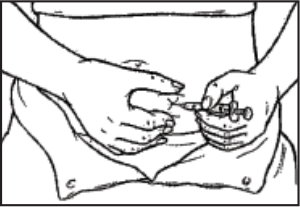
- Hold the syringe at a right angle to the skin, like a dart. Quickly insert the needle all the way into the skin fold.
- Push the plunger of the syringe down with a steady motion. Keep pushing until all the f uid is injected into the skin.
- Release the skin fold and pull the needle straight out.
- You should inject BRAVELLE® into the skin on your abdomen a few inches below your navel, to the left or right. Each day, inject at a different spot to help reduce soreness. For example, on day 1, inject yourself at spot 1. The next day, use spot 2. And so on. On the fifth day, begin again with spot 1.
-
Disposing of the syringe and needle
- Put the cap back on the needle. Place the needle and syringe in the large jar for used supplies.
- Get rid of any opened sterile diluent or medicine still left in the vial. Used vials can be put in the large jar.
- After your pregnancy treatment plan is over, be sure to get rid of the large jar. Ask your doctor about the best way to do this.
-
After the injection
- If there is any bleeding, place a small piece of gauze over the injection spot. Apply gentle pressure to stop the bleeding.
- If the injection spot becomes sore, put some ice on it. Keep the ice on the spot for a minute or so, and then remove it. Do this 3 or 4 times.
How should I store BRAVELLE®?
- Store BRAVELLE® in the refrigerator at 2º to 8ºC (36º to 46ºF) or at room temperature, 20º to 25ºC (68º to 77º F). Do not freeze. Protect from light
- Use BRAVELLE® right away after mixing it with sterile diluent.
- Keep BRAVELLE® and all medicines out of the reach of children.
General information about BRAVELLE®
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information leaflets. Do not use BRAVELLE® for a condition for which it was not prescribed. Do not give BRAVELLE® to other people, even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about BRAVELLE®. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about BRAVELLE® that is written for health professionals.
You can also call 1-888-FERRING.
MANUFACTURED FOR:
FERRING PHARMACEUTICALS INC.
PARSIPPANY, NJ 07054
6317-01
09/08
| BRAVELLE
urofollitropin kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021289 | 05/06/2002 | |
| Labeler - Ferring Pharmaceuticals Inc. (103722955) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ferring GmbH - Kiel | 328609615 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| F.M. Howell & Company | 962888116 | pack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Jubilant HollisterStier General Partnership | 246762764 | manufacture | |
Revised: 02/2012 Ferring Pharmaceuticals Inc.