oramorph sr (Morphine Sulfate) tablet
[Xanodyne pharmaceuticals, inc.]
CII
Rx only
| NOTE | |
| THIS IS A SUSTAINED RELEASE DOSAGE FORM. PATIENT SHOULD BE INSTRUCTED TO SWALLOW THE TABLET AS A WHOLE; THE TABLET SHOULD NOT BE BROKEN IN HALF, NOR SHOULD IT BE CRUSHED OR CHEWED. THE SUSTAINED RELEASE OF MORPHINE FROM ORAMORPH SR SHOULD BE TAKEN INTO CONSIDERATION IN EVENT OF ADVERSE REACTIONS OR OVERDOSAGE. |
DESCRIPTION
Each tablet for oral administration contains:
Morphine sulfate . . . . . . . . . . 15 mg, 30 mg, 60 mg, or 100 mg in a tablet that provides for sustained release of the medication.
Morphine sulfate occurs as white, feathery, silky crystals, cubical masses of crystals, or white crystalline powder; it is soluble in water and slightly soluble in alcohol. Morphine has a pKa of 7.9, with an octanol/water partition coefficient of 1.42 at pH 7.4. At this pH, the tertiary amino group is mostly ionized, making the molecule water-soluble. Morphine is significantly more water-soluble than any other opioid in clinical use.
Chemically, morphine sulfate is 7,8-didehydro-4,5α-epoxy-17-methyl-morphinian-3,6α-diol sulfate (2:1)(salt) pentahydrate, and has the following structural formula:
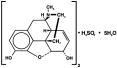
Each ORAMORPH SR Tablet contains 15 mg, 30 mg, 60 mg, or 100 mg Morphine Sulfate USP. Inactive ingredients: Lactose, Hydroxypropyl Methylcellulose, Colloidal Silicon Dioxide, and Stearic Acid.
CLINICAL PHARMACOLOGY
Morphine is the prototype of many narcotic drugs that interact predominantly with the opioid μ-receptor. These μ-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas. They are also found on the terminal axons of primary afferents within laminae I and II (substantia gelatinosa) of the spinal cord and in the spinal nucleus of the trigeminal nerve.
In clinical settings, morphine exerts its principal pharmacological effect on the central nervous system and gastrointestinal tract. Its primary actions of therapeutic value are analgesia and sedation. Morphine appears to increase the patient's tolerance for pain and to decrease discomfort, although the presence of the pain itself may still be recognized. In addition to analgesia, alterations in mood, euphoria and dysphoria, and drowsiness commonly occur.
Morphine depresses various respiratory centers, depresses the cough reflex, and constricts the pupils. Analgesically effective blood levels of morphine may cause nausea and vomiting directly by stimulating the chemoreceptor trigger zone, but nausea and vomiting are significantly more common in ambulatory than in recumbent patients, as is postural syncope.
Morphine increases the tone and decreases the propulsive contractions of the smooth muscle of the gastrointestinal tract. The resultant prolongation in gastrointestinal transit time is responsible for the constipating effect of morphine. Because morphine may increase biliary-tract pressure, some patients with biliary colic may experience worsening rather than relief of pain.
While morphine generally increases the tone of urinary-tract smooth muscle, the net effect tends to be variable, in some cases producing urinary urgency, in others, difficulty in urination.
In therapeutic doses, morphine does not usually exert major effects on the cardiovascular system. Some patients, however, exhibit a propensity to develop orthostatic hypotension and fainting. Rapid intravenous injection is more likely to precipitate a fall in blood pressure than oral dosing.
Morphine can cause histamine release, which appears to be responsible for dilation of cutaneous blood vessels, with resulting flushing of the face and neck, pruritus, and sweating.
PHARMACOKINETICS
ORAMORPH SR Tablets are a sustained release oral dosage form of morphine sulfate. Only about 40% of the administered dose reaches the central compartment because of first-pass effect (i.e., metabolism in the gut wall and liver). Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen and brain. Morphine also crosses the placental membrane and has been found in breast milk.
For all practical purposes, virtually all morphine is converted to glucuronide metabolites; only a small fraction (less than 5%) of absorbed morphine is demethylated. Among these glucuronide metabolites, morphine-3-glucuronide is present in the highest plasma concentration following oral administration; a smaller fraction is converted to morphine-6-glucuronide, which has the greater analgesic activity of these two metabolites.
The glucuronide system has a high capacity and is not easily saturated, even in disease. Therefore, the rate of delivery of morphine to the gut and liver does not influence the total and/or the relative quantities of the various metabolites formed.
The pharmacokinetic parameters following oral administration of ORAMORPH SR, presented in the table below, show considerable inter-subject variation, but are representative of average values reported in the literature. The volume of distribution (Vd) for morphine is 4 liters per kilogram (L/kg), and the terminal elimination half-life is approximately 2 to 4 hours.
| Pharmacokinetic Parameter {scientific notation} (unit) | Dose of ORAMORPH SR | ||||
| Dose of 2 x 15 mg | 30 mg | 60 mg | 100 mg | ||
|
Dose metabolized = approximately 90% |
|||||
|
Morphine metabolites (%) = morphine-3-glucuronide (55-75%), |
|||||
|
morphine-6-glucuronide (1-5%) |
|||||
|
1Derived from pharmacokinetic studies in 24 normal volunteers |
|||||
| Bioavailability
(oral compared to injectable) | approximately 40% | ||||
| Time-to-peak plasma concentration {Tmax} (h) | mean (range) | 3.7 (1-6) | 3.8 (1-7) | 3.8 (2-7) | 3.6 (1.5-12) |
| Peak plasma concentration {Cmax}
(ng/mL) [single dose] | mean (range) | 11.1 (6.5-16.2) | 9.9 (5.0-18.6) | 16.1 (10.0-25.3) | 27.4 (14.1-46.1) |
| Volume of distribution (calculated from mean clearance and terminal half-life) {Vd(β)} (L/kg) | mean | - - - - - - - 4 L/kg - - - - - - - | |||
Following the administration of conventional, immediate-release, oral morphine products, approximately 50% of the morphine, that will ever reach the central compartment, reaches it within 30 minutes. Following the administration of an equal amount of ORAMORPH SR to normal volunteers, however, 50% of absorption occurs, on average, after 1.5 hours.
A pharmacokinetic study in normal volunteers indicates that there is little to no effect on the systemic bioavailability of ORAMORPH SR when administered with food.
Although variation in the physico-mechanical properties of a formulation of an oral morphine drug product can affect both its absolute bioavailability and its absorption rate constant (ka), morphine distribution and clearance are unchanged, as they are fundamental properties of morphine in the organism. However, in chronic use, the possibility of shifts in metabolite-to-parent drug ratios cannot be excluded.
When immediate-release oral morphine or ORAMORPH SR is given on a fixed dosing regimen, steady-state is achieved in about one to two days.
For a given dose and dosing interval, the Area-Under-the-Curve (AUC) and average blood concentration of morphine at steady-state (Css) will be independent of the type of oral formulation administered, as long as the formulations have the same absolute bioavailability. The absorption rate of a formulation will, however, affect the maximum (Cmax) and minimum (Cmin) plasma concentrations and the time between administration and their occurrence. For any fixed dose and dosing interval, ORAMORPH SR will have, at steady-state, a lower Cmax and a higher Cmin than conventional immediate-release morphine, which might be a therapeutic advantage in chronic pain control (see also PHARMACODYNAMICS).
The clearance of morphine occurs primarily as renal excretion of morphine-3-glucuronide. A small amount of the glucuronide conjugate is excreted in the bile, and there is some minor enterohepatic recycling; about 10% of the glucuronide conjugate is excreted in the feces. Because morphine is essentially metabolized in the liver, the effects of renal disease on morphine's clearance are not likely to be pronounced. As with any drug, however, caution should be taken to guard against unanticipated accumulation if renal and/or hepatic function is seriously impaired.
PHARMACODYNAMICS
In clinical settings, morphine's primary actions of therapeutic value are analgesia and sedation. Opiate analgesia involves at least three anatomical areas of the central nervous system: the periaqueductal-periventricular gray matter, the ventromedial medulla, and the spinal cord. Morphine appears to increase the patient's tolerance for pain, and to decrease the discomfort, although the presence of pain itself may still be recognized.
While there is considerable variability in the relationship between morphine blood concentration and analgesic response, effective analgesia probably will not occur below some minimum blood level in a given patient. The minimum effective blood level for analgesia will vary among patients, especially among patients who have been previously treated with potent μ-agonist opioids. Similarly, there is considerable variability in the relationship between morphine plasma concentration and untoward clinical responses, but higher concentrations are more likely to be toxic.
In contrast to immediate-release morphine, after dosing with ORAMORPH SR, the morphine blood levels show reduced fluctuation between peak and trough plasma levels; that means that they are more centered within the theoretical 'therapeutic window'. On the other hand, the reduced fluctuation in morphine plasma concentration might conceivably affect other phenomena, as for example, the rate of tolerance induction.
ORAMORPH SR is an analgesic intended for patients who require chronic morphine analgesia and who will have, in consequence, markedly different degrees of pharmacodynamic tolerance for opioid drugs. Morphine and similar opioids induce tolerance to their effects, so that a shortening of the duration of satisfactory analgesia may be the first sign of an increase in tolerance.
Once patients are started on morphine, the dose required for satisfactory analgesia will rise, with the rate of development of tolerance varying, depending on the patient's prior narcotic use, level of pain, degree of anxiety, use of other CNS-active drugs, circulatory status, total daily dose, and the dosing interval.
INDICATIONS AND USAGE
ORAMORPH SR is indicated for the relief of pain in patients who require opioid analgesics for more than a few days.
CONTRAINDICATIONS
ORAMORPH SR is contraindicated in patients with respiratory depression in the absence of resuscitative equipment, in patients with acute or severe bronchial asthma and in patients with known hypersensitivity to morphine.
ORAMORPH SR is contraindicated in any patient who has or is suspected of having a paralytic ileus.
WARNINGS
IMPAIRED RESPIRATION:
Respiratory depression is the chief hazard of all morphine preparations. Respiratory depression occurs more frequently in the elderly and debilitated patients, as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Morphine should be used with extreme caution in patients who have a decreased respiratory reserve (e.g., emphysema, severe obesity, kyphoscoliosis, or paralysis of the phrenic nerve). ORAMORPH SR should not be given in cases of chronic asthma, upper airway obstruction, or in any other chronic pulmonary disorder without due consideration of the known risk of acute respiratory failure following morphine administration in such patients.
DRUG ABUSE AND DEPENDENCE - CONTROLLED SUBSTANCE:
Morphine sulfate is a Schedule II narcotic under the United States Controlled Substance Act (21 U.S.C. 801-886).
Morphine is the most commonly cited prototype for narcotic substances that possess an addiction-forming or addiction-sustaining liability. A patient may be at risk for developing a dependence to morphine if used improperly or for overly long periods of time. As with all potent opioids which are μ-agonists, tolerance as well as psychological and physical dependence to morphine may develop irrespective of the route of administration (oral, intravenous, intramuscular, intrathecal, or epidural). Individuals with a prior history of opioid or other substance abuse or dependence, being more apt to respond to euphorogenic and reinforcing properties of morphine, would be considered to be at greater risk.
Care must be taken to avert withdrawal symptoms when morphine is discontinued abruptly or upon administration of a narcotic antagonist.
PRECAUTIONS
General Precautions:
Selection of patients for treatment with ORAMORPH SR should be governed by the same principles that apply to the use of morphine or other potent opioid analgesics. Narcotic analgesics are drugs that have a narrow therapeutic index in the old, the sick, and the infirm, i.e., the very population in which their use is indicated. Physicians should individualize treatment with ORAMORPH SR in every case, weighing the need for analgesia against the risks of serious or fatal reactions to the drug.
Use in Patients with Increased Intracranial Pressure or with Head Injury:
ORAMORPH SR should be used with extreme caution in patients with increased intracranial pressure or with head injury. The respiratory depressant effects of morphine (increased pCO2) may result in elevation of cerebrospinal fluid pressure and may thus be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increased intracranial pressure. Morphine produces effects which may obscure neurologic signs of further increases in pressure in patients with head injuries. Pupillary changes (miosis), associated with morphine, may conceal the existence, extent, and course of intracranial pathology.
Use in Hepatic or Renal Disease:
The clearance of morphine may be reduced in patients with hepatic dysfunction, while the clearance of its metabolites may be decreased in renal dysfunction. This will be manifested by both a prolonged elimination half-life and the accumulation of levels of either morphine or its metabolites in excess of those produced in normals, with the potential for an increase of adverse effects (see WARNINGS and ADVERSE REACTIONS). These changes in morphine pharmacodynamics, in patients with hepatic or renal dysfunctions, should be considered when adjusting the dose and dosage intervals, taking also into account the slow-release character of ORAMORPH SR.
Drug Interactions:
Use with Other Central Nervous System Depressants:
The depressant effects of morphine are potentiated by the presence of other CNS depressants such as alcohol, sedatives, antihistaminics, or psychotropic drugs. Use of neuroleptics in conjunction with oral morphine may increase the risk of respiratory depression, hypotension and profound sedation or coma.
Interaction with Mixed Agonist/Antagonist Opioid Analgesics:
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, or buprenorphine) should NOT be administered to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic. In these patients, the mixed agonist/antagonist may alter the analgesic effect or may precipitate withdrawal symptoms.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies of morphine sulfate in animals to evaluate the drug's carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy:
Teratogenic Effects - Category C: There are no well-controlled studies in women, but marketing experience does not include any evidence of adverse effects on the fetus following routine (short-term) clinical use of morphine sulfate products. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus.
ORAMORPH SR should be used in pregnant women only when clearly needed. (See also: PRECAUTIONS: Labor and Delivery, and DRUG ABUSE AND DEPENDENCE CONTROLLED SUBSTANCE.)
Nonteratogenic Effects:
Infants born from mothers who have been taking morphine chronically may exhibit withdrawal symptoms.
Labor and Delivery:
ORAMORPH SR is not recommended for use in women during and immediately prior to labor. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contractions.
Neonates, whose mothers received opioid analgesics during labor, should be observed closely for signs of respiratory depression. A specific narcotic antagonist, naloxone, should be available for reversal of narcotic-induced respiratory depression in the neonate.
Nursing Mothers:
ORAMORPH SR should not be given to nursing mothers because morphine is excreted in maternal milk. Effects on the nursing infant are not known, but withdrawal symptoms can occur in breast-fed infants when maternal administration of morphine sulfate is stopped.
Pediatric Use:
ORAMORPH SR has not been evaluated in children. Its use in the pediatric population is, therefore, not recommended.
Geriatric Use:
The pharmacodynamic effects of morphine in the aged are more variable than in the younger population. Patients will vary widely in the effective initial dose, rate of development of tolerance, and the frequency and magnitude of associated adverse effects as the dose is increased. Individualization of doses must receive careful attention in elderly patients.
Information for Patients:
If clinically advisable, patients receiving ORAMORPH SR brand of morphine sulfate sustained release tablets, should be given the following instructions by the physician:
- Morphine may produce psychological and/or physical dependence. For this reason, the dose of the drug should not be increased without consulting a physician.
- Morphine may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery).
- Morphine should not be taken with alcohol or other CNS depressants (sleep aids, tranquilizers) because additive effects, including CNS depression, may occur. A physician should be consulted if other prescription and/or over-the-counter medications are currently being used or are prescribed for future use.
- For women of childbearing potential, who become or are planning to become pregnant, a physician should be consulted regarding analgesics and other drug use.
ADVERSE REACTIONS
| NOTE: THE SUSTAINED RELEASE OF MORPHINE FROM ORAMORPH SR SHOULD BE TAKEN INTO CONSIDERATION IN THE EVENT OF OCCURRING ADVERSE REACTIONS. |
Adverse reactions caused by morphine are essentially those observed with other opioid analgesics. They include the following major hazards: respiratory depression, and less frequently, circulatory depression, apnea, shock and cardiac arrest secondary to respiratory and/or circulatory depression.
Most Frequently Observed Reactions:
Constipation, nausea, vomiting, lightheadedness, dizziness, sedation, dysphoria, euphoria, and sweating. Some of these effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain. Some adverse reactions in ambulatory patients may be alleviated if the patient is in a supine position.
Less Frequently Observed Reactions:
Body as a Whole: Edema, antidiuretic effect, chills, muscle tremor, muscle rigidity.
Cardiovascular: Flushing of the face, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension.
Gastrointestinal: Dry mouth, biliary tract spasm, laryngospasm, anorexia, diarrhea, cramps, taste alterations.
Genitourinary: Urine retention or hesitance, reduced libido and/or potency.
Nervous System: Weakness, headache, agitation, tremor, uncoordinated muscle movements, seizure, paresthesia, alterations of mood (nervousness, apprehension, depression, floating feelings), dreams, transient hallucination and disorientation, visual disturbances, insomnia, increased intracranial pressure.
Skin: Pruritus, urticaria and other skin rashes.
Special Senses: Blurred vision, nystagmus, diplopia, miosis.
DRUG ABUSE AND DEPENDENCE
Opioid analgesics may cause psychological and physical dependence (see WARNINGS). Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug, or these symptoms may be precipitated through the administration of drugs with antagonistic activity, e.g., naloxone or mixed agonist/antagonist analgesics (pentazocine, etc.; see also OVERDOSAGE). Physical dependence usually does not occur, to a clinically significant degree, until several weeks of continued opioid usage. Tolerance, in which increasingly larger doses are required to produce the same degree of analgesia, is initially manifested by a shortened duration of analgesic effect and, subsequently, by decreases in the intensity of analgesia. In patients with chronic pain, as well as in opioid-tolerant cancer patients, the administration of ORAMORPH SR (morphine sulfate) should be guided by the degree of tolerance manifested. Physical dependence, per se, is not ordinarily a concern when one is dealing with opioid-tolerant patients whose pain and suffering is associated with an irreversible illness.
If ORAMORPH SR is abruptly discontinued, an abstinence syndrome may occur. Withdrawal symptoms, in patients dependent on morphine, begin shortly before the time of the next scheduled dose, reaching a peak at 36 to 72 hours after the last dose, and then slowly subside over a period of 7 to 10 days. Symptoms include yawning, sweating, lacrimation, rhinorrhea, restless sleep, dilated pupils, gooseflesh, irritability, tremor, nausea, vomiting, and diarrhea.
Treatment of the abstinence syndrome is primarily symptomatic and supportive, including maintenance of proper fluid and electrolyte balance. If withdrawal has inadvertently been precipitated in a patient who requires narcotics for pain management, the withdrawal syndrome can be terminated rapidly by the administration of an appropriate dose of a pure agonist opioid, such as morphine. The degree of physical dependence of a patient on ORAMORPH SR can be intentionally reduced by a gradual reduction of dosage and symptomatic treatment of withdrawal symptomatology.
OVERDOSAGE
| NOTE: THE SUSTAINED RELEASE OF MORPHINE FROM ORAMORPH SR SHOULD BE TAKEN INTO CONSIDERATION IN THE EVENT OF AN OVERDOSAGE. |
Overdosage of morphine is characterized by respiratory depression, with or without concomitant CNS depression. Since respiratory arrest may result either through direct depression of the respiratory center, or as the result of hypoxia, primary attention should be given to the establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted, or controlled, ventilation. The narcotic antagonist, naloxone, is a specific antidote. An initial dose of 0.4 to 2 mg of naloxone should be administered intravenously, simultaneously with respiratory resuscitation. If the desired degree of counteraction and improvement in respiratory function is not obtained, naloxone may be repeated at 2 to 3 minute intervals. If no response is observed after 10 mg of naloxone has been administered, the diagnosis of narcotic-induced, or partial narcotic-induced, toxicity should be questioned. Intramuscular or subcutaneous administration may be used if the intravenous route is not available.
As the duration of effect of naloxone is considerably shorter than that of ORAMORPH SR, repeated administration may be necessary. Patients should be closely observed for evidence of renarcotization.
| NOTE: In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Use of a narcotic antagonist in such a person should be avoided. If necessary to treat serious respiratory depression in a physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist. |
When indicated, gut decontamination should be performed via emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) with cathartic. Since ORAMORPH SR is a sustained release product, absorption may be expected to continue for many hours, particularly following an overdose, combined with decreased peristaltic activity of the gastrointestinal tract.
Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
DOSAGE AND ADMINISTRATION
(See also: CLINICAL PHARMACOLOGY, WARNINGS and PRECAUTIONS sections.)
NOTE: ORAMORPH SR TABLET MUST BE SWALLOWED WHOLE. DO NOT BREAK THE TABLET IN HALF. DO NOT CRUSH OR CHEW. TAKING BROKEN, CHEWED OR CRUSHED TABLETS COULD LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF MORPHINE.
ORAMORPH SR is intended for use in patients who require more than several days of continuous treatment with a potent opioid analgesic. The sustained release nature of the formulation allows it to be administered on a more convenient schedule than conventional immediate-release oral morphine products (see CLINICAL PHARMACOLOGY - PHARMACOKINETICS). However, ORAMORPH SR does not release morphine continuously over the course of a dosing interval. The administration of single doses of ORAMORPH SR on a q12h dosing schedule will result in peak and trough plasma levels similar to those following an identical daily dose of morphine administered using conventional oral formulations on a q4h regimen. If pain is not controlled for a full 12 hours, then the dosing interval should be shortened, but to no less than 8 hours.
As with any potent opioid, it is critical to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. Attention should be given to the following in determining the initial dose of ORAMORPH SR, (1) the daily dose, potency and characteristics of a pure agonist, or mixed agonist-antagonist, the patient has been taking previously, (2) the reliability of the relative potency estimate to calculate the dose of morphine needed [N.B.: potency estimates may vary with the route of administration], (3) the fact that roughly only 40% of the morphine sulfate in ORAMORPH SR becomes available after pre-systemic metabolization in the intestinal wall and the liver, (4) the degree of opioid tolerance, and (5) the general condition and medical status of the patient.
The following dosing recommendations for ORAMORPH SR, therefore, can only be considered suggested approaches to the series of clinical decisions in the management of pain of an individual patient.
Conversion from Conventional Immediate-Release Oral Morphine to ORAMORPH SR:
A patient's daily morphine requirement is established by using the Daily Oral Morphine Requirement of the immediate-release formulation which gives the Daily Oral Morphine Requirement for ORAMORPH SR. Since ORAMORPH SR is given on an 'every 12 hour' schedule, the single dose of ORAMORPH SR is half of the Daily Oral Morphine Requirement. Dose and dosing interval is adjusted as needed (see discussion below). For initial conversion, the 30 mg tablet strength is recommended for patients with a daily morphine requirement of 120 mg or less.
Conversion from Parenteral Morphine or Other Opioid Analgesics (parenteral or oral) to ORAMORPH SR:
Because of uncertainty about relative estimates of opioid potency and cross tolerance, as well as intersubject variation, initial dosing regimens should be conservative, i.e., an underestimation of the 24-hour oral morphine requirement is preferred to an overestimate. To this end, initial individual doses of ORAMORPH SR should be estimated conservatively. In patients whose daily morphine requirements are expected to be less than or equal to 120 mg per day, the 30 mg tablet strength is recommended for the initial titration period. Once a stable dose regimen is reached, the patient can be converted to the 60 mg or 100 mg tablet strength, as appropriate.
Estimates of the relative potency of opioids are only approximate, and are influenced by route of administration, individual patient differences, and possibly, by the patient's medical condition. Consequently, it is difficult to recommend any precise rule for converting a patient to ORAMORPH SR directly. However, the following general points should be considered:
- Parenteral to oral morphine ratio: Estimates of the oral-to-parenteral potency of morphine vary. Some authorities suggest that a dose of morphine only 3 times the daily parenteral morphine requirement may be sufficient in chronic use settings. (3 times the Daily Parenteral Morphine Requirement = the Daily Oral Morphine Requirement)
- Other parenteral or oral opioids to oral morphine: Because of a lack of reliable relative potency assays, specific recommendations are not possible. In general, it is safer to underestimate the Total Daily Dose of ORAMORPH SR required and rely upon ad hoc supplementation to deal with inadequate analgesia (see discussion which follows).
Use of ORAMORPH SR as the First Opioid Analgesic:
There has been no systematic evaluation of ORAMORPH SR as an initial opioid analgesic in the management of pain. Because it may be more difficult to titrate a patient using a sustained release morphine, it is ordinarily advisable to begin treatment using an immediate release formulation.
Considerations in the Adjustment of Dosing Regimens:
Whatever the approach, if signs of excessive opioid effects are observed early in a dosing interval, the next dose should be reduced. If this adjustment leads to inadequate analgesia, i.e., 'breakthrough' pain occurs late in the dosing interval, the dosing interval may be shortened. Alternatively, a supplemental dose of a short-acting analgesic may be given.
As experience is gained, adjustments can be made to obtain an appropriate balance between pain relief, opioid side effects and the convenience of the dosing schedule.
In adjusting dose requirements, it is recommended that the dosing interval never be extended beyond 12 hours, because the administration of very large single doses of ORAMORPH SR may lead to acute overdosage.
For patients with low daily morphine requirements, the 15 mg tablet should be used. In this regard, adjustment in dose should NOT be attempted by breaking or crushing the tablets. ORAMORPH SR tablets are intended to be swallowed whole.
Conversion from ORAMORPH SR to Parenteral Opioids:
When converting a patient from ORAMORPH SR to parenteral opioids, it is best to assume that the parenteral to oral potency relationship is high. NOTE THAT THIS IS THE CONVERSE OF THE STRATEGY USED WHEN THE DIRECTION OF CONVERSION IS FROM THE PARENTERAL TO ORAL FORMULATIONS. IN BOTH CASES, HOWEVER, THE AIM IS TO ESTIMATE THE NEW DOSE CONSERVATIVELY. For example, to estimate the required 24-hour dose of morphine for IM use, one could employ a conversion of 1 mg of morphine IM for every 6 mg of morphine as ORAMORPH SR. Of course, the IM 24-hour dose would have to be divided by six and administered on a q4h regimen. This approach is recommended because it is least likely to cause overdosage.
HOW SUPPLIED
ORAMORPH® SR (Morphine Sulfate)
Sustained Release Tablets are available as follows:
15 mg white tablets (Identified 54 782)
[Embossed with 15]
NDC 66479-540-25: Unit dose, 25 tablets per card (reverse numbered), 4 cards per shipper.
NDC 66479-540-10: Bottles of 100 tablets.
30 mg white tablets (Identified 54 409)
[Embossed with 30]
NDC 66479-541-25: Unit dose, 25 tablets per card (reverse numbered), 4 cards per shipper.
NDC 66479-541-10: Bottles of 100 tablets.
60 mg white tablets (Identified 54 933)
[Embossed with 60]
NDC 66479-542-25: Unit dose, 25 tablets per card (reverse numbered), 1 card per shipper.
NDC 66479-542-10: Bottles of 100 tablets.
100 mg white tablets (Identified 54 862)
[Embossed with 100]
NDC 66479-543-25: Unit dose, 25 tablets per card (reverse numbered), 1 card per shipper.
NDC 66479-543-10: Bottles of 100 tablets.
DEA Order Form Required.
Dispense in a tight, light-resistant container.
Store at 25°C (77°F); excursions are permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]
Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.
Safety and Handling Instructions:
ORAMORPH SR is supplied as tablets that pose little risk of direct exposure to health care personnel and should be handled and disposed of in accordance with hospital policy. Patients and their families should be instructed to dispose of ORAMORPH SR tablets, that are no longer needed, down the toilet.
ORAMORPH is a trademark of Xanodyne Pharmaceuticals, Inc.
© 2006 Xanodyne Pharmaceuticals, Inc.
Marketed by:
Xanodyne pharmaceuticals, inc.
Newport, KY 41071
10004432/01
Rev. 02-2006
| Oramorph SR (Morphine Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Oramorph SR (Morphine Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Oramorph SR (Morphine Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Oramorph SR (Morphine Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 05/2007Xanodyne pharmaceuticals, inc.