LIPOSYN III
-
soybean oil,
egg phospholipids and
glycerin injection, emulsion
Hospira, Inc.
----------
DESCRIPTION
Liposyn III 30% (Intravenous Fat Emulsion) is a sterile, nonpyrogenic fat emulsion for intravenous administration. The Pharmacy Bulk Package is a sterile dosage form which contains multiple single doses for use only in a pharmacy bulk admixture program.
Liposyn III 30% contains 30% soybean oil, 1.8% egg phosphatides and 2.5% glycerin in water for injection. Sodium hydroxide has been added for pH adjustment. pH 8.4 (6.0 to 9.0). Liposyn III 30% has an osmolarity of 293 mOsmol/L (actual) and a specific gravity of 0.985. The total caloric value of Liposyn III 30% including fat, phospholipid and glycerol is 2.9 kcal/mL. Of this total, approximately 1.5 kcal/mL is supplied by linoleic acid.
Liposyn III 30% contains emulsified fat particles of approximately 0.4 micron in diameter, similar to naturally occurring chylomicrons.
This Pharmacy Bulk Package is intended for use in the preparation of 3-in-1 or total nutrient admixtures (TNAs) in a pharmacy admixture program.
Soybean Oil, USP is a mixture of neutral triglycerides with the following structure:
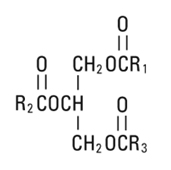
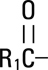 ,
, 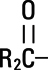 and
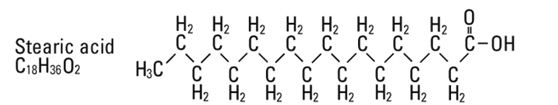
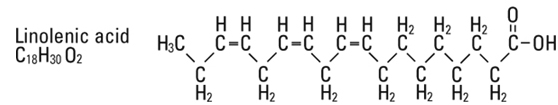
and 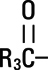 are saturated and unsaturated fatty acid residues. The major component fatty acids are approximately 54.5% linoleic, 22.4% oleic, 10.5% palmitic, 4.2% stearic, and 8.3% linolenic acid. These fatty acids have the following chemical and structural formulas:
are saturated and unsaturated fatty acid residues. The major component fatty acids are approximately 54.5% linoleic, 22.4% oleic, 10.5% palmitic, 4.2% stearic, and 8.3% linolenic acid. These fatty acids have the following chemical and structural formulas:
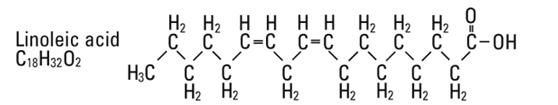
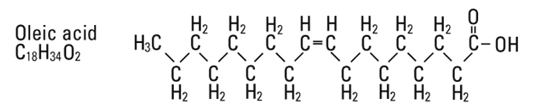
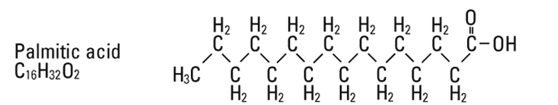
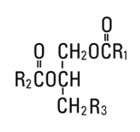
Egg phosphatides, purified, are primarily a mixture of naturally occurring phospholipids which are isolated from the egg yolk. These phospholipids have the following general structure:
 and
and 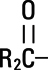 are the same saturated and unsaturated fatty acid residues that abound in neutral fats. R3 is primarily either the choline [HOCH2CH2N(CH3)3OH] ester or ethanolamine (HOCH2CH2NH2) ester of phosphoric acid (H3PO4).
are the same saturated and unsaturated fatty acid residues that abound in neutral fats. R3 is primarily either the choline [HOCH2CH2N(CH3)3OH] ester or ethanolamine (HOCH2CH2NH2) ester of phosphoric acid (H3PO4).
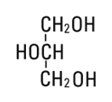
Glycerin, USP is chemically designated C3H8O3 and is a clear, colorless, hygroscopic syrupy liquid. It has the following structural formula:
CLINICAL PHARMACOLOGY
Liposyn III (intravenous fat emulsion) provides the patient requiring parenteral nutrition with a source of calories and the essential fatty acids normally obtained from a nutritionally complete oral diet. The supplemental polyunsaturated fat prevents biochemical changes of essential fatty acid deficiency (EFAD) and prevents and reverses EFAD clinical manifestations (e.g., scaliness of skin, growth retardation, poor wound healing and sparse hair growth).
The infused fat particles are cleared from the bloodstream in a manner thought to be similar to the clearing of chylomicrons. Following infusion, there is a transient increase in plasma triglycerides. The triglycerides are hydrolyzed to free fatty acids and glycerol by the enzyme, lipoprotein lipase. The free fatty acids either enter the tissues (where they may be oxidized or resynthesized into triglycerides and stored) or circulate in the plasma, bound to albumin. In the liver, circulating free fatty acids are oxidized or converted to very low density lipoproteins that re-enter the bloodstream.
Phosphatides are the hydrophobic components of membranes and provide electrically insulated layers. They are involved in the formation of membrane structures. Choline prevents the deposition of fat in the liver.
Glycerol is metabolized to carbon dioxide and glycogen or is used in the synthesis of body fats.
INDICATIONS AND USAGE
Liposyn III 30% Pharmacy Bulk Package is indicated for use in a pharmacy admixture program for the preparation of 3-in-1 or total nutrient admixtures (TNAs).
Liposyn III (intravenous fat emulsion) is indicated as a source of calories for patients requiring parenteral nutrition. Where such nutrition is required for extended periods of time (more than 5 days), Liposyn III is also indicated as a source of essential fatty acids to prevent or reverse biochemical changes in fatty acid composition of plasma lipids (elevated triene/tetraene ratio) and the clinical manifestations of EFAD.
CONTRAINDICATIONS
LIPOSYN III 30% PHARMACY BULK PACKAGE IS NOT INTENDED FOR DIRECT INTRAVENOUS ADMINISTRATION. DILUTING LIPOSYN III 30% TO A 10% OR 20% CONCENTRATION WITH AN INTRAVENOUS FLUID SUCH AS NORMAL SALINE OR OTHER DILUENT DOES NOT PRODUCE A DILUTION THAT IS EQUIVALENT IN COMPOSITION TO LIPOSYN III 10% OR 20% I.V. FAT EMULSIONS, AND SUCH A DILUTION SHOULD NOT BE GIVEN BY DIRECT INTRAVENOUS ADMINISTRATION (e.g., THROUGH A Y-CONNECTOR).
The administration of Liposyn III (intravenous fat emulsion) is contraindicated in patients demonstrating disturbances in normal fat metabolism such as pathologic hyperlipemia, lipoid nephrosis or acute pancreatitis if accompanied by hyperlipemia.
Partly used containers must not be stored for later use. Do not use any bottle in which there appears to be an oiling out of the emulsion.
WARNINGS
Deaths in preterm infants after infusion of intravenous fat emulsions have been reported in the medical literature.1,2 Autopsy findings included intravascular fat accumulation in the lungs. Treatment of premature and low birth weight infants with intravenous fat emulsion must be based upon careful benefit-risk assessment. Strict adherence to the recommended total daily dose is mandatory; hourly infusion rate should be as slow as possible in each case and should not in any case exceed 1 g/kg in four hours. Premature and small for gestational age infants have poor clearance of intravenous fat emulsion and increased free fatty acid plasma levels following fat emulsion infusion; therefore, serious consideration must be given to administration of less than the maximum recommended doses in these patients in order to decrease the likelihood of intravenous fat overload. The infant’s ability to eliminate infused fat from the circulation must be carefully monitored (such as triglyceride and/or plasma free fatty acid levels). The lipemia must clear between daily infusions.
Precipitation occurring in parenteral nutrition admixtures as a result of incorrect admixture practices has been reported. Precipitate formation may occur as a result of incompatibility of calcium and phosphate salts. Liposyn III 30%, as a component of these admixtures, will obscure the presence of particulate matter (see DOSAGE AND ADMINISTRATION and MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION).
Caution should be exercised in administering Liposyn III (intravenous fat emulsion) to patients with severe liver damage, pulmonary disease, anemia or blood coagulation disorders or when there is danger of fat embolism. The too rapid administration of Liposyn III can cause fluid and/or fat overloading resulting in dilution of serum electrolyte concentrations, over-hydration, congested states, pulmonary edema, impaired pulmonary diffusion capacity or metabolic acidosis.
Caution should be exercised when admixing Liposyn III 30% (Intravenous Fat Emulsion). Studies have documented the stability of Liposyn III 30%, when admixed with Hospira electrolytes, Hospira trace metals, and Hospira Dextrose Injection, USP and Hospira Aminosyn II (amino acid) Injection. (Also see MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION under DOSAGE AND ADMINISTRATION). These compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal of refrigeration. Admixtures should be stored at room temperature (25°C) during this period. Reference should be made to the individual package inserts for detailed information on each component.
The prime destabilizers of emulsions are excessive acidity (low pH) and inappropriate electrolyte content. Careful consideration should be given to the dosage levels of the divalent cations (Ca++ and Mg++) and phosphates administered, as these have been shown to cause emulsion instability. Amino acid solutions exert a buffering effect, protecting the emulsion. NOTE: The TPN admixture containers used in the stability studies were formulated to minimize lipid/container interactions. The principal bag materials were a nonphthalate polyvinylchloride (PVC) or the CR3 co-polyester material of which the Nutrimix® dual-chamber flexible container is comprised. CR3 contains no plasticizers.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
PRECAUTIONS
Because free fatty acids displace bilirubin bound to albumin, the use of lipid infusions in jaundiced or premature infants should be undertaken with caution.
During Liposyn III (intravenous fat emulsion) administration, the patient’s hemogram, blood coagulation, liver function, platelet count and plasma lipid profile must be closely monitored. The lipemia must clear between daily infusions. Liposyn III should be discontinued should a significant abnormality in any one of these parameters be attributed to the infusion.
It is recommended that a 1.2 micron air-eliminating filter be used during administration of admixtures containing Liposyn III 30%.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility of Liposyn III have not been conducted.
Pregnancy Category C. Animal reproduction studies have not been conducted with Liposyn III. It is also not known whether Liposyn III can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Liposyn III should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in milk, caution should be exercised when Liposyn III 30% is administered to a nursing mother.
Do not dispense the contents of any container in which the emulsion appears to be oiling out.
AVOID OVERDOSAGE ABSOLUTELY.
ADVERSE REACTIONS
Sepsis due to contamination of administration equipment and thrombophlebitis due to vein irritation from concurrently administered hypertonic solutions have been encountered. These are attributable to I.V. therapy in general or to the type of infusion administered.
Adverse reactions directly related to fat emulsions are of two types: (1) immediate (acute) and (2) long term (chronic). In studies of lipid products in general, the following immediate reactions have been noted: Allergic reactions, hyperlipemia, dyspnea, cyanosis, flushing, dizziness, headache, sleepiness, nausea, vomiting, hyperthermia, sweating, chest and back pain, thrombocytopenia (rarely in neonates), hypercoagulability and transient increases in liver enzymes.
The following reactions have been noted with long-term therapy with lipid infusions in general: Hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leucopenia, transient increases in liver function tests, overloading syndrome and the deposition of brown pigment (“fat pigment”) in the reticuloendothelial tissue of the liver. The significance of this last occurrence and its cause are unknown.
If symptoms and signs of acute respiratory distress develop, appropriate medical intervention should be instituted immediately and the cause of the distress investigated. The parenteral nutrition infusion should be replaced with a dextrose infusion (to prevent rebound hypoglycemia) and checked for the presence of particulate matter and oiling out of the emulsion (see MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION).
OVERDOSAGE
In the event of fat overload during therapy, stop the infusion of Liposyn III until visual inspection of the plasma, determination of triglyceride concentrations, or measurement of plasma light-scattering activity by nephelometry indicates the lipid has cleared. Re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
DOSAGE AND ADMINISTRATION
Liposyn III (intravenous fat emulsion) should be administered as part of 3-in-1 or total nutrient admixture via peripheral vein or central venous catheter.
Adult Patients
The initial infusion rate of the nutrient admixture in adults should be the equivalent of 0.1 g fat/minute for the first 15 to 30 minutes of infusion. If no untoward reactions occur (see ADVERSE REACTIONS section), the infusion rate of the nutrient admixture can be increased to be equivalent to 0.2 g fat/minute. For adults, the admixture should not contain more than 330 mL of Liposyn III 30% on the first day of therapy. If the patient has no untoward reactions, the dose can be increased on the following day. The daily dosage should not exceed 2.5 g of fat/kg of body weight (8.3 mL of Liposyn III 30% per kg). Liposyn III 30% should make up no more than 60% of the total caloric input to the patient. Carbohydrate and a source of amino acids should comprise the remaining caloric input.
Pediatric Patients
The dosage for premature infants starts at 0.5 g fat/kg body weight/24 hours (1.7 mL Liposyn III 30%) and may be increased in relation to the infant’s ability to eliminate fat. The maximum dosage recommended by the American Academy of Pediatrics is 3 g fat/kg/24 hours.3
The initial rate of infusion of the nutrient admixture in older pediatric patients should be no more than 0.01 g fat/minute for the first 10 to 15 minutes. If no untoward reactions occur, the rate can be changed to permit infusion of 0.1 g of fat/kg/hour. The daily dosage should not exceed 3 g of fat/kg of body weight.3 Liposyn III 30% should make up no more than 60% of the total caloric input to the patient. Carbohydrate and a source of amino acids should comprise the remaining caloric input.
Essential Fatty Acid Deficiency
When Liposyn III 30% is administered to correct essential fatty acid deficiency, eight to ten percent of the caloric input should be supplied by Liposyn III 30% in order to provide adequate amounts of linoleic acids. When EFAD occurs together with stress, the amount of Liposyn III 30% needed to correct the deficiency may be increased.
Administration
See MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION section for information regarding mixing this fat emulsion with other parenteral fluids.
It is recommended that a 1.2 micron air-eliminating filter be used during administration of admixtures containing Liposyn III 30%.
Admixtures were compounded in a nonphthalate polyvinylchloride (PVC) flexible container (see NOTE under WARNINGS). See MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION. Studies have documented the stability of Liposyn III 30%, when admixed with Hospira electrolytes, Hospira trace metals, Hospira Dextrose Injection, USP and Hospira Aminosyn II (amino acid) Injection. These compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal of refrigeration. Admixtures should be stored at room temperature (25°C) during this period.
Conventional administration sets contain polyvinylchloride (PVC) components that have DEHP (diethylhexyl phthalate) as a plasticizer. Fat-containing fluids such as Liposyn III extract DEHP from this PVC component, and it may be advisable to consider infusion of 3-in-1 admixtures containing Liposyn III 30% through a non-DEHP administration set.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
MIXING INSTRUCTIONS FOR COMBINED ADMINISTRATION
Caution should be exercised when admixing Liposyn III (Intravenous Fat Emulsion).
It is absolutely essential that the admixture be prepared using strict aseptic technique, as this nutrient mixture is a good growth medium for microorganisms.
These compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal of refrigeration. Admixtures should be stored at room temperature (25°C) during this period. Reference should be made to the individual package inserts for detailed information on each component.
Because warming parenteral nutrition admixtures may contribute to the formation of precipitates, once administration begins, care should be taken to avoid excessive warming of the admixture.
Because of the potential for life-threatening events, caution should be taken to ensure that precipitates do not form in any parenteral nutrition admixture. Precipitates can develop because of a number of factors such as: the concentration, pH and phosphate content of the amino acid solution, the calcium and phosphate additives or the order of mixing. The presence of a lipid emulsion in the TPN admixture will obscure the presence of any precipitate.
Admixtures must be made using specific mixing protocols for 2-in-1 and 3-in-1 admixtures. Each pharmacy mixing protocol should be verified for compatibility of the resulting admixture. Admixtures should be verified at the final concentration used for amino acids, dextrose, lipid emulsion, the specific additives and the specific order of addition. Different manufacturers’ components should not be substituted in a tested pharmacy admixture protocol without prior verification of compatibility.
When adding calcium and/or phosphate to parenteral nutrition solutions, the pharmacist must assess the impact of the following factors on the formation of a precipitate: 1) order of mixing, 2) salt form and concentration of electrolytes, 3) concentration of amino acids, 4) concentration of dextrose, 5) concentration of lipid emulsion, 6) temperature and pH and 7) presence of other additives.
The amounts of phosphate and of calcium added to the admixture are critical. The solubility of the added calcium should be calculated from the volume at the time the calcium is added. It should not be based upon the final volume. Any phosphate ions present in other constituents and the volume at the time the phosphate is added should be considered when calculating the amount of phosphate additives. Also, when adding calcium and phosphate to an admixture, the phosphate should be added first. The calcium should not be added consecutively or in close sequence to the phosphate addition.
Appropriate techniques should be used to ensure adequate mixing of all constituents. The admixture should be carefully inspected for the presence of precipitates.
The following proper mixing sequence must be followed for manual mixing to minimize pH-related problems by ensuring that typically acidic dextrose injections are not mixed with lipid emulsion alone:
-
Transfer all of the aqueous components of the 3-in-1 admixture into the pooling container first, including the dextrose, amino acid solution, electrolytes and trace metals.
-
Add the Liposyn III 30%.
-
Add the multivitamin supplement last, immediately prior to the start of patient infusion.
Admixing should be accompanied by gentle agitation to avoid localized concentration effects. NOTE: Simultaneous or sequential mixing of Liposyn III 30% with other nutritional substrates using an automated pumping system is considered an acceptable method for admixture compounding 3-in-1 admixtures.
Recommended Directions for Use of the Pharmacy Bulk Package
Use aseptic technique
-
Perform all manipulation in an appropriate laminar flow hood.
-
Aseptically remove aluminum overseal.
-
Insert piercing pin of sterile transfer set and suspend unit in a laminar flow hood. Insertion of a piercing pin into the outlet port should be performed only once in a Pharmacy Bulk Package solution. ONCE THE OUTLET SITE HAS BEEN ENTERED, THE WITHDRAWAL OF CONTAINER CONTENTS SHOULD BE COMPLETED PROMPTLY IN ONE CONTINUOUS OPERATION. Should this not be possible, a maximum time of 4 hours from transfer set pin or implement insertion is permitted to complete fluid transfer operations, i.e., discard container no later than 4 hours after initial closure puncture. Date and time of container entry should be noted on the area designated on the container label.
-
Sequentially dispense aliquots of Liposyn III 30% into I.V. containers using appropriate transfer set.
During fluid transfer operations, the Pharmacy Bulk Package should be maintained under the storage conditions recommended in the labeling.
HOW SUPPLIED
Liposyn III (intravenous fat emulsion) 30% is a white to slightly off-white emulsion with no evidence of oiling out of the emulsion.
Liposyn III 30% in the Pharmacy Bulk Package (List No. 6892) is supplied in 500 mL glass bottles.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
References
-
Levene M, Wigglesworth J, Desai R. Pulmonary fat accumulation after Intralipid infusion in the preterm infant.
Lancet II: 815-818, (Oct. 18), 1980.
-
Dahms B, Halpin T. Pulmonary arterial lipid deposit in newborn infants receiving intravenous lipid infusion.
J. Pediatrics: 97:800-805, (Nov.), 1980.
-
American Academy of Pediatrics: Use of intravenous fat emulsion in pediatric patients.
Pediatrics 1981; 68:5 (Nov.):738-43.
Revised: August, 2005
©Hospira 2005 EN-1012 Printed in USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
| LIPOSYN III
soybean oil, egg phospholipids, and glycerin injection, emulsion |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020181 | 05/01/2011 | 06/01/2011 |
| Labeler - Hospira, Inc. (141588017) |
Revised: 03/2012 Hospira, Inc.