PROLOPRIM
-
trimethoprim tablet
Monarch Pharmaceuticals, Inc
----------
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Proloprim Tablets and other antibacterial drugs. Proloprim Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
PROLOPRIM (trimethoprim) is a synthetic antibacterial available in tablet form for oral administration. Each scored white tablet contains 100 mg trimethoprim and the inactive ingredients corn starch, lactose, magnesium stearate, and sodium starch glycolate. Each scored yellow tablet contains 200 mg trimethoprim and the inactive ingredients corn starch, D & C Yellow No. 10, magnesium stearate, and sodium starch glycolate.
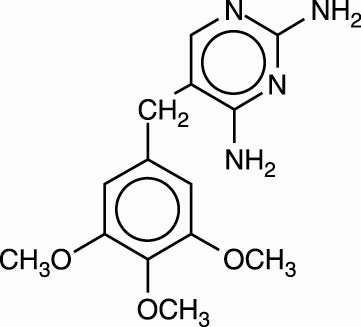
Trimethoprim is 5-[(3,4,5,-trimethoxyphenyl)methyl]-2,4-pyrimidinediamine. It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.32 and the molecular formula C14H18N4O3. The structural formula is:
CLINICAL PHARMACOLOGY
Trimethoprim is rapidly absorbed following oral administration. It exists in the blood as unbound, protein-bound, and metabolized forms. Ten to twenty percent of trimethoprim is metabolized, primarily in the liver; the remainder is excreted unchanged in the urine. The principal metabolites of trimethoprim are the 1- and 3-oxides and the 3’- and 4’- hydroxy derivatives. The free form is considered to be the therapeutically active form. Approximately 44% of trimethoprim is bound to plasma proteins.
Mean peak serum concentrations of approximately 1.0 mcg/mL occur 1 to 4 hours after oral administration of a single 100-mg dose. A single 200-mg dose will result in serum levels approximately twice as high. The half-life of trimethoprim ranges from 8 to 10 hours. However, patients with severely impaired renal function exhibit an increase in the half-life of trimethoprim, which requires either dosage regimen adjustment or not using the drug in such patients (see DOSAGE AND ADMINISTRATION ). During a 13-week study of trimethoprim administered at a daily dosage of 200 mg (50 mg qid), the mean minimum steady-state concentration of the drug was 1.1 mcg/mL. Steady-state concentrations were achieved within 2 to 3 days of chronic administration and were maintained throughout the experimental period.
Excretion of trimethoprim is primarily by the kidneys through glomerular filtration and tubular secretion. Urine concentrations of trimethoprim are considerably higher than are the concentrations in the blood. After a single oral dose of 100 mg, urine concentrations of trimethoprim ranged from 30 to 160 mcg/mL during the 0- to 4-hour period and declined to approximately 18 to 91 mcg/mL during the 8- to 24-hour period. A 200 mg single oral dose will result in trimethoprim urine levels approximately twice as high. After oral administration, 50% to 60% of trimethoprim is excreted in the urine within 24 hours, approximately 80% of this being unmetabolized trimethoprim.
Since normal vaginal and fecal flora are the source of most pathogens causing urinary tract infections, it is relevant to consider the distribution of trimethoprim into these sites. Concentrations of trimethoprim in vaginal secretions are consistently greater than those found simultaneously in the serum, being typically 1.6 times the concentrations of simultaneously obtained serum samples. Sufficient trimethoprim is excreted in the feces to markedly reduce or eliminate trimethoprim-susceptible organisms from the fecal flora.
Trimethoprim also passes the placental barrier and is excreted in human milk.
Microbiology: Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. This binding is much stronger for the bacterial enzyme than for the corresponding mammalian enzyme. Thus, trimethoprim selectively interferes with bacterial biosynthesis of nucleic acids and proteins.
In vitro serial dilution tests have shown that the spectrum of antibacterial activity of trimethoprim includes the common urinary tract pathogens with the exception of Pseudomonas aeruginosa.
The dominant non-Enterobacteriaceae fecal organisms, Bacteroides spp. and Lactobacillus spp., are not susceptible to trimethoprim concentrations obtained with the recommended dosage.
Trimethoprim has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms
Staphylococcus species (coagulase-negative strains, including S. saprophyticus )
Aerobic gram-negative microorganisms
Enterobacter species
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Susceptibility Testing Methods
Dilution techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of trimethoprim powder. The MIC values should be interpreted according to the following criteria:
For testing Enterobacteriaceae and Staphylococcus spp.:
| MIC (mcg/mL) | Interpretation |
| ≤ 8 | Susceptible (S) |
| ≥ 16 | Resistant (R) |
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard trimethoprima powder should provide the following MIC values:
| Microorganism | MIC (mcg/mL) | |
| Escherichia coli | ATCC 25922 | 0.5–2.0 |
| Staphylococcus aureus | ATCC 29213 | 1.0–4.0 |
a Very medium-dependent.
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5-mcg trimethoprim to test the susceptibility of microorganisms to trimethoprim.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5-mcg trimethoprim disk should be interpreted according to the following criteria:
For testing Enterobacteriaceae and Staphylococcus spp.:
| Zone Diameter (mm) | Interpretation |
| ≥ 16 | Susceptible (S) |
| 11–15 | Intermediate (I) |
| ≤ 10 | Resistant (R) |
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC of trimethoprim.
As with standardized dilution techniques, diffusion methods require the use of the laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 5-mcg trimethoprim disk should provide the following zone diameters in these laboratory test quality control strains:
| Microorganism | MIC (mcg/mL) | |
| Escherichia coli | ATCC 25922 | 0.5–2.0 |
| Microorganism | Zone Diameter (mm) | |
| Escherichia coli | ATCC 25922 | 21–28 |
| Staphylococcus aureus | ATCC 25923 | 19–26 |
b Mueller-Hinton agar should be checked for excessive levels of thymidine. To determine whether Mueller-Hinton medium has sufficiently low levels of thymidine and thymine, an Enterococcus faecalis (ATCC 29212 or ATCC 33186) may be tested with trimethoprim/sulfamethoxazole disks. A zone of inhibition ≥ 20 mm that is essentially free of fine colonies indicates a sufficiently low level of thymidine and thymine.
INDICATIONS AND USAGE
For the treatment of initial episodes of uncomplicated urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species, and coagulase-negative Staphylococcus species, including S. saprophyticus.
Cultures and susceptibility tests should be performed to determine the susceptibility of the bacteria to trimethoprim. Therapy may be initiated prior to obtaining the results of these tests.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Proloprim Tablets and other antibacterial drugs. Proloprim Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
PROLOPRIM is contraindicated in individuals hypersensitive to trimethoprim and in those with documented megaloblastic anemia due to folate deficiency.
WARNINGS
Serious hypersensitivity reactions have been reported rarely in patients on trimethoprim therapy. Trimethoprim has been reported rarely to interfere with hematopoiesis, especially when administered in large doses and/or for prolonged periods.
The presence of clinical signs such as sore throat, fever, pallor, or purpura may be early indications of serious blood disorders (see OVERDOSAGE: Chronic).
Complete blood counts should be obtained if any of these signs are noted in a patient receiving trimethoprim and the drug discontinued if a significant reduction in the count of any formed blood element is found.
PRECAUTIONS
General:
Trimethoprim should be given with caution to patients with possible folate deficiency. Folates may be administered concomitantly without interfering with the antibacterial action of trimethoprim. Trimethoprim should also be given with caution to patients with impaired renal or hepatic function (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Prescribing Proloprim Tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Drug Interactions:
PROLOPRIM may inhibit the hepatic metabolism of phenytoin. Trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 51% and decreased the phenytoin metabolic clearance rate by 30%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Drug/Laboratory Test Interactions:
Trimethoprim can interfere with a serum methotrexate assay as determined by the Competitive Binding Protein Technique (CBPA) when a bacterial dihydrofolate reductase is used as the binding protein. No interference occurs, however, if methotrexate is measured by a radioimmunoassay (RIA).
The presence of trimethoprim may also interfere with the Jaffé alkaline picrate reaction assay for creatinine, resulting in overestimations of about 10% in the range of normal values.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenesis:
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with trimethoprim.
Mutagenesis:
Trimethoprim was demonstrated to be nonmutagenic in the Ames assay. In studies at two laboratories, no chromosomal damage was detected in cultured Chinese hamster ovary cells at concentrations approximately 500 times human plasma levels; at concentrations approximately 1000 times human plasma levels in these same cells, a low level of chromosomal damage was induced at one of the laboratories. No chromosomal abnormalities were observed in cultured human leukocytes at concentrations of trimethoprim up to 20 times human steady-state plasma levels. No chromosomal effects were detected in peripheral lymphocytes of human subjects receiving 320 mg of trimethoprim in combination with up to 1600 mg of sulfamethoxazole per day for as long as 112 weeks.
Pregnancy:
Teratogenic Effects:
Pregnancy Category C. Trimethoprim has been shown to be teratogenic in the rat when given in doses 40 times the human dose. In some rabbit studies, the overall increase in fetal loss (dead and resorbed and malformed conceptuses) was associated with doses six times the human therapeutic dose.
While there are no large, well-controlled studies on the use of trimethoprim in pregnant women, Brumfitt and Pursell,4 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or trimethoprim in combination with sulfamethoxazole. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving trimethoprim and sulfamethoxazole. There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received trimethoprim and sulfamethoxazole at the time of conception or shortly thereafter.
Because trimethoprim may interfere with folic acid metabolism, PROLOPRIM should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
Trimethoprim is excreted in human milk. Because trimethoprim may interfere with folic acid metabolism, caution should be exercised when PROLOPRIM is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in pediatric patients below the age of 2 months have not been established. The effectiveness of trimethoprim as a single agent has not been established in pediatric patients under 12 years of age.
Geriatric Use:
Clinical studies of Proloprim (trimethoprim) Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience4,5 has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Case reports of hyperkalemia in elderly patients receiving trimethoprim-sulfaethoxazole have been published.6 Trimethoprim is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor potassium concentrations and to monitor renal function by calculating creatinine clearance.
Information for Patients:
Patients should be counseled that antibacterial drugs including Proloprim Tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Proloprim Tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Proloprim Tablets or other antibacterial drugs in the future.
ADVERSE REACTIONS
The adverse effects encountered most often with trimethoprim were rash and pruritus.
Dermatologic:
Rash, pruritus, and phototoxic skin eruptions. At the recommended dosage regimens of 100 mg b.i.d. or 200 mg q.d., each for 10 days, the incidence of rash is 2.9% to 6.7%. In clinical studies which employed high doses of PROLOPRIM, an elevated incidence of rash was noted. These rashes were maculopapular, morbilliform, pruritic, and generally mild to moderate, appearing 7 to 14 days after the initiation of therapy.
Hypersensitivity:
Rare reports of exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell Syndrome), and anaphylaxis have been received.
Gastrointestinal:
Epigastric distress, nausea, vomiting, and glossitis. Elevation of serum transaminase and bilirubin has been noted, but the significance of this finding is unknown. Cholestatic jaundice has been rarely reported.
OVERDOSAGE
Acute:
Signs of acute overdosage with trimethoprim may appear following ingestion of 1 gram or more of the drug and include nausea, vomiting, dizziness, headaches, mental depression, confusion, and bone marrow depression (see Chronic subsection).
Treatment consists of gastric lavage and general supportive measures. Acidification of the urine will increase renal elimination of trimethoprim. Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating the drug.
Chronic:
Use of trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia, and/or megaloblastic anemia. If signs of bone marrow depression occur, trimethoprim should be discontinued and the patient should be given leucovorin; 5 to 15 mg leucovorin daily has been recommended by some investigators.
DOSAGE AND ADMINISTRATION
The usual oral adult dosage is 100 mg of PROLOPRIM every 12 hours or 200 mg PROLOPRIM every 24 hours, each for 10 days. The use of trimethoprim in patients with a creatinine clearance of less than 15 mL/min is not recommended. For patients with a creatinine clearance of 15 to 30 mL/min, the dose should be 50 mg every 12 hours.
HOW SUPPLIED
100-mg Tablets (white, scored, round-shaped), containing 100 mg trimethoprim–bottle of 100 (NDC 61570-057-01). Imprint on tablets “PROLOPRIM 09A.” Store at 15° to 25°C (59° to 77°F) in a dry place.
200-mg Tablets (yellow, scored, round-shaped), containing 200 mg trimethoprim–bottle of 100 (NDC 61570-058-01). Imprint on tablets “PROLOPRIM 200.” Store at 15° to 25°C (59° to 77°F) in a dry place and protect from light.
Rx Only.
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 3rd ed.; Approved Standard. NCCLS Document M7-A4, Vol. 17, No. 2. NCCLS, Wayne, PA, January, 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. Sixth Edition. Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1, NCCLS, Wayne, PA, January, 1997.
- Brumfitt W, Pursell R. Trimethoprim-sulfamethoxazole in the treatment of bacteriuria in women. J Infect Dis . 1973;128(suppl):S657-S663.
- Lacey RW, Simpson MHC, Fawcett C, et al. Comparison of single-dose trimethoprim with a five-day course for the treatment of urinary tract infections in the elderly. Age and Ageing 10: 179–185, 1981.
- Ewer TC, Bailey RR, Gilchrist NL, et al. Comparative study of norfloxacin and trimethoprim for the treatment of elderly patients with urinary tract infection. NZ Med J 101: 537–539, 1988.
- Marinella MA. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontology 45: 209–212, 1999.
Prescribing Information as of January 2006.
Distributed by: Monarch Pharmaceuticals, Inc. Bristol, TN 37620
(A wholly owned subsidiary of King Pharmaceuticals, Inc.)
Manufactured by: DSM Pharmaceuticals, Inc. Greenville, NC 27834
| PROLOPRIM
trimethoprim tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017943 | 07/14/1982 | 01/31/2007 |
| PROLOPRIM
trimethoprim tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017943 | 07/14/1982 | 01/31/2007 |
| Labeler - Monarch Pharmaceuticals, Inc (809587413) |
Revised: 09/2011 Monarch Pharmaceuticals, Inc

