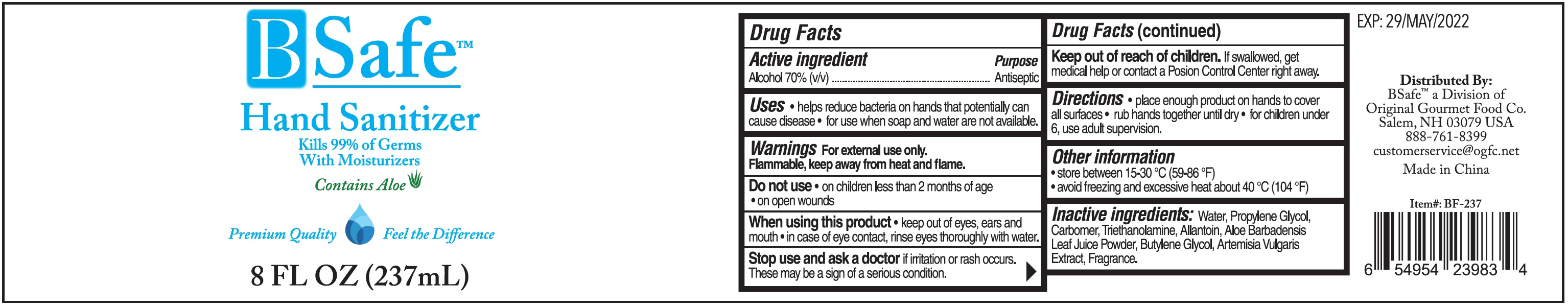
BSAFE HAND SANITIZER- alcohol gel
Original Gourmet Food Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BSafe Hand Sanitizer
Uses
- helps reduce bacteria on hands that potentially can cause disease
- for use when soap and water are not available.
Warnings
For external use only. Flammable, keep away from heat and flame.
Directions
- place enough product on hands to cover all surfaces
- rub hands together until dry.
- for children under 6, use adult supervision.
Other information
- store between 15-30 °C (59-86 °F)
- avoid freezing and excessive heat about 40 °C (104 °F)
| BSAFE HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Original Gourmet Food Company (164757184) |
Revised: 3/2023
Document Id: f794887f-6eec-1047-e053-6294a90ad8df
Set id: a8fd5469-c9f2-46c1-e053-2995a90a53c6
Version: 3
Effective Time: 20230323
Original Gourmet Food Company