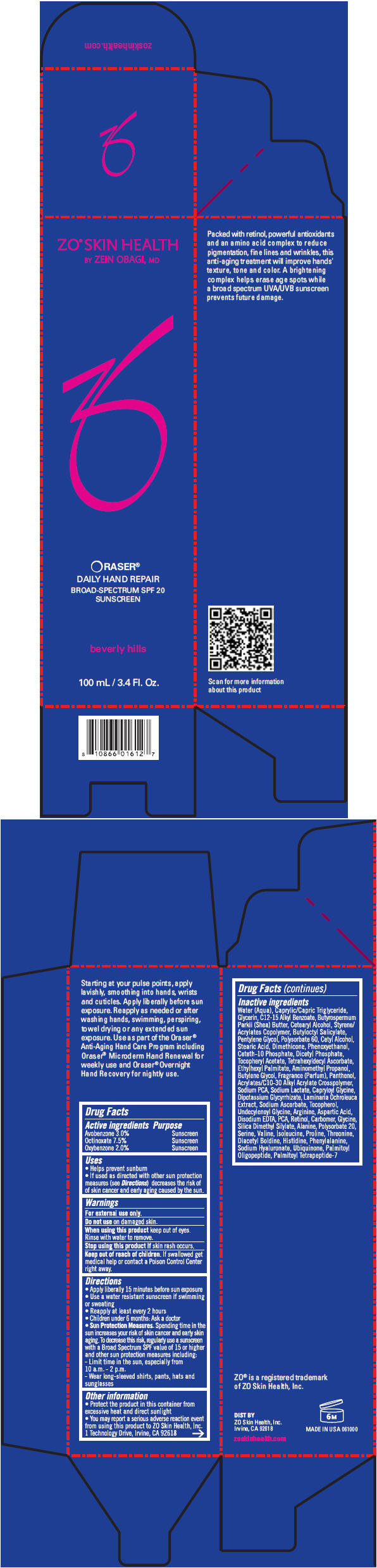
ORASER DAILY HAND REPAIR BROAD-SPECTRUM SPF 20 SUNSCREEN- avobenzone, octinoxate, and oxybenzone lotion
ZO Skin Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ORASER
® DAILY HAND REPAIR Broad-Spectrum SPF 20 Sunscreen
National Drug Code: 42851-050
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
Other information
- Protect the product in this container from excessive heat and direct sunlight
- You may report a serious adverse reaction event from using this product to ZO Skin Health, Inc. 1 Technology Drive, Irvine, CA 92618
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglyceride, Glycerin, C12-15 Alkyl Benzoate, Butyrospermum Parkii (Shea) Butter, Cetearyl Alcohol, Styrene/Acrylates Copolymer, Butyloctyl Salicylate, Pentylene Glycol, Polysorbate 60, Cetyl Alcohol, Stearic Acid, Dimethicone, Phenoxyethanol, Ceteth-10 Phosphate, Dicetyl Phosphate, Tocopheryl Acetate, Tetrahexyldecyl Ascorbate, Ethylhexyl Palmitate, Aminomethyl Propanol, Butylene Glycol, Fragrance (Parfum), Panthenol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Sodium PCA, Sodium Lactate, Capryloyl Glycine, Dipotassium Glycyrrhizate, Laminaria Ochroleuca Extract, Sodium Ascorbate, Tocopherol, Undecylenoyl Glycine, Arginine, Aspartic Acid, Disodium EDTA, PCA, Retinol, Carbomer, Glycine, Silica Dimethyl Silylate, Alanine, Polysorbate 20, Serine, Valine, Isoleucine, Proline, Threonine, Diacetyl Boldine, Histidine, Phenylalanine, Sodium Hyaluronate, Ubiquinone, Palmitoyl Oligopeptide, Palmitoyl Tetrapeptide-7
| ORASER DAILY HAND REPAIR BROAD-SPECTRUM SPF 20 SUNSCREEN
avobenzone, octinoxate, and oxybenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ZO Skin Health, Inc. (826468527) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pak Lab | 177711082 | manufacture(42851-050) | |