BASF CARES ANTISEPTIC HAND RUB- isopropyl alcohol solution
BASF Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
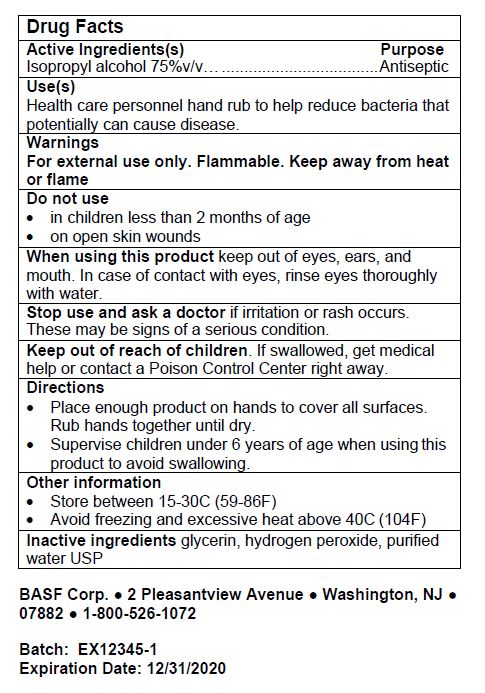
DRUG FACTS
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| BASF CARES ANTISEPTIC HAND RUB
isopropyl alcohol solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - BASF Corporation (014403088) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BASF Corporation | 003000049 | api manufacture(10117-2121) | |
Revised: 1/2023
Document Id: ce37d257-a1ec-4c82-ae36-c62593c9cd69
Set id: 767f509f-c5ee-4dd1-b170-874a3021ba1e
Version: 2
Effective Time: 20230101
BASF Corporation