EFINACONAZOLE- efinaconazole solution/ drops
Padagis US LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EFINACONAZOLE TOPICAL SOLUTION safely and effectively. See full prescribing information for EFINACONAZOLE TOPICAL SOLUTION. EFINACONAZOLE topical solution Initial U.S. Approval: 2014 INDICATIONS AND USAGEEfinaconazole topical solution is an azole antifungal indicated for the topical treatment of onychomycosis of the toenail(s) due to Trichophyton rubrum and Trichophyton mentagrophytes. (1) DOSAGE AND ADMINISTRATION |
DOSAGE FORMS AND STRENGTHSSolution: 10%. (3) CONTRAINDICATIONSNone. (4) ADVERSE REACTIONSThe most common adverse reactions (incidence >1%) were ingrown toenails, application site dermatitis, application site vesicles, and application site pain. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Padagis at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 5/2022 |
FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS6 ADVERSE REACTIONS6.1 Clinical Trials Experience6.2 Postmarketing Experience7 DRUG INTERACTIONS8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.2 Lactation8.4 Pediatric Use8.5 Geriatric Use |
11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics12.4 Microbiology13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION
|
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Efinaconazole topical solution, 10% is an azole antifungal indicated for the topical treatment of onychomycosis of the toenail(s) due to Trichophyton rubrum and Trichophyton mentagrophytes.
2 DOSAGE AND ADMINISTRATION
Apply efinaconazole topical solution to affected toenails once daily for 48 weeks, using the integrated flow-through brush applicator. When applying efinaconazole topical solution, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered.
Efinaconazole topical solution is for topical use only and not for oral, ophthalmic, or intravaginal use.
3 DOSAGE FORMS AND STRENGTHS
Efinaconazole topical solution, 10% contains 100 mg of efinaconazole in each gram of clear, colorless to pale yellow solution.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two clinical trials, 1227 subjects were treated with efinaconazole topical solution, 1161 for at least 24 weeks and 780 for 48 weeks. Adverse reactions reported within 48 weeks of treatment and in at least 1% of subjects treated with efinaconazole topical solution and those reported in subjects treated with the vehicle are presented in Table 1.
Table 1: Adverse Reactions Reported by at Least 1% of Subjects Treated for up to 48 Weeks
|
Adverse Event, n (%) |
Efinaconazole Topical Solution N = 1227 |
Vehicle N = 413 |
|
Ingrown toenail |
28 (2.3%) |
3 (0.7%) |
|
Application site dermatitis |
27 (2.2%) |
1 (0.2%) |
|
Application site vesicles |
20 (1.6%) |
0 (0.0%) |
|
Application site pain |
13 (1.1%) |
1 (0.2%) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of efinaconazole topical solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
General Disorders and Administration Site Conditions: Application site erythema and exfoliation
Skin and Subcutaneous Tissue Disorders: Onychomadesis, Nail discoloration
7 DRUG INTERACTIONS
In vitro studies have shown that efinaconazole topical solution, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available human data for the use of efinaconazole topical solution during pregnancy to inform any drug associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, efinaconazole did not cause malformations or any harm to the fetus when administered to pregnant rabbits and rats during the period of organogenesis at subcutaneous doses up to 112 and 154 times, respectively, the Maximum Recommended Human Dose (MRHD) based on Area Under the Curve (AUC) comparisons. Embryolethality was observed only in rats in the presence of maternal toxicity at systemic exposures 559 times the MRHD based on AUC comparisons. Subcutaneous efinaconazole administration to pregnant rats from the beginning of organogenesis through the end of lactation did not cause embryofetal toxicity or developmental effects at systemic exposures 17 times the MRHD based on AUC comparisons (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 2, 10 and 50 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-16) to pregnant female rats. In the presence of maternal toxicity, embryofetal toxicity (increased embryofetal deaths, decreased number of live fetuses, and placental effects) was noted at 50 mg/kg/day (559 times the MRHD based on AUC comparisons). No embryofetal toxicity was noted at 10 mg/kg/day (112 times the MRHD based on AUC comparisons). No malformations were observed at 50 mg/kg/day (559 times the MRHD based on AUC comparisons).
Subcutaneous doses of 1, 5, and 10 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-19) to pregnant female rabbits. In the presence of maternal toxicity, there was no embryofetal toxicity or malformations at 10 mg/kg/day (154 times the MRHD based on AUC comparisons).
In a pre-and postnatal development study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day efinaconazole were administered from the beginning of organogenesis (gestation day 6) through the end of lactation (lactation day 20). In the presence of maternal toxicity, embryofetal toxicity (increased prenatal pup mortality, reduced live litter sizes and increased postnatal pup mortality) was noted at 25 mg/kg/day. No embryofetal toxicity was noted at 5 mg/kg/day (17 times the MRHD based on AUC comparisons). No effects on postnatal development were noted at 25 mg/kg/day (89 times the MRHD based on AUC comparisons).
8.2 Lactation
Risk Summary
It is not known whether efinaconazole is excreted in human milk. After repeated subcutaneous administration, efinaconazole was detected in milk of nursing rats. Because many drugs are excreted in human milk, caution should be exercised when efinaconazole topical solution is administered to nursing women.
The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for efinaconazole topical solution, and any potential adverse effects on the breastfed infant from efinaconazole topical solution.
8.4 Pediatric Use
Safety and effectiveness of efinaconazole topical solution in pediatric subjects under 6 years of age have not been established.
Pediatric use information is approved for Bausch Health Americas, Inc.’s Jublia (efinaconazole) topical solution. However, due to Bausch Health Americas, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Of the total number of subjects in clinical trials of efinaconazole topical solution, 11.3% were 65 and over, while none were 75 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and the younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
11 DESCRIPTION
Efinaconazole topical solution, 10% is a clear, colorless to pale yellow solution for topical use. Each gram of efinaconazole topical solution contains 100 mg of efinaconazole. Efinaconazole is an azole antifungal with a chemical name of (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl) butan-2-ol. The structural formula for efinaconazole is represented below:
Molecular Formula: C18H22F2N4O Molecular Weight: 348.39
Efinaconazole topical solution contains the following inactive ingredients: alcohol (55.8% v/v), anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Efinaconazole topical solution is an azole antifungal [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Systemic absorption of efinaconazole in 18 adult subjects with severe onychomycosis was determined after application of efinaconazole topical solution once daily for 28 days to patients’ 10 toenails and 0.5 cm adjacent skin. The concentration of efinaconazole in plasma was determined at multiple time points over the course of 24-hour periods on days 1, 14, and 28. Efinaconazole mean ± SD plasma Cmax on Day 28 was 0.67 ± 0.37 ng/mL and the mean ± SD AUC was 12.15 ± 6.91 ng*h/mL. The plasma concentration versus time profile at steady state was generally flat over a 24-hour dosing interval. In a separate study of healthy volunteers, the plasma half-life of efinaconazole following daily applications when applied to all 10 toenails for 7 days was 29.9 hours.
Specific Populations
Pediatric patients
Pediatric use information is approved for Bausch Health Americas, Inc.’s Jublia (efinaconazole) topical solution. However, due to Bausch Health Americas, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
Drug Interactions
Efinaconazole topical solution is considered a non-inhibitor of the CYP450 enzyme family. In in vitro studies using human liver microsomes, efinaconazole did not inhibit CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2PE1 and CYP3A4 enzyme activities at expected clinical systemic concentrations. In vitro studies in human primary hepatocytes showed that efinaconazole did not induce CYP1A2 or CYP3A4 activities.
12.4 Microbiology
Mechanism of Action
Efinaconazole is an azole antifungal. Efinaconazole inhibits fungal lanosterol 14α-demethylase involved in the biosynthesis of ergosterol, a constituent of fungal cell membranes.
Activity In Vitro and In Vivo
Efinaconazole has been shown to be active against isolates of the following microorganisms, both in vitro and in clinical infections. Efinaconazole exhibits in vitro minimum inhibitory concentrations (MICs) of 0.06 mcg/mL or less against most (≥90%) isolates of the following microorganisms:
Trichophyton rubrum
Trichophyton mentagrophytes
Mechanism of Resistance
Efinaconazole drug resistance development was studied in vitro against T. mentagrophytes, T. rubrum and C. albicans. Serial passage of fungal cultures in the presence of sub-growth inhibitory concentrations of efinaconazole increased the MIC by up to 4-fold. The clinical significance of these in vitro results is unknown.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year dermal carcinogenicity study in mice was conducted with daily topical administration of 3%, 10% and 30% efinaconazole solution. Severe irritation was noted at the treatment site in all dose groups, which was attributed to the vehicle and confounded the interpretation of skin effects by efinaconazole. The high dose group was terminated at week 34 due to severe skin reactions. No drug-related neoplasms were noted at doses up to 10% efinaconazole solution (248 times the MRHD based on AUC comparisons).
Efinaconazole revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster lung cell chromosome aberration assay) and one in vivo genotoxicity test (mouse peripheral reticulocyte micronucleus assay).
No effects on fertility were observed in male and female rats that were administered subcutaneous doses up to 25 mg/kg/day efinaconazole (279 times the MRHD based on AUC comparisons) prior to and during early pregnancy. Efinaconazole delayed the estrous cycle in females at 25 mg/kg/day but not at 5 mg/kg/day (56 times MRHD based on AUC comparisons).
14 CLINICAL STUDIES
The safety and efficacy of once daily use of efinaconazole topical solution for the treatment of onychomycosis of the toenail were assessed in two 52-week prospective, multicenter, randomized, double-blind clinical trials in subjects 18 years and older (18 to 70 years of age) with 20% to 50% clinical involvement of the target toenail, without dermatophytomas or lunula (matrix) involvement. The trials compared 48 weeks of treatment with efinaconazole topical solution to the vehicle solution. The Complete Cure rate was assessed at Week 52 (4 weeks after completion of therapy). Complete cure was defined as 0% involvement of the target toenail (no clinical evidence of onychomycosis of the target toenail) in addition to Mycologic Cure, defined as both negative fungal culture and negative KOH. Table 2 lists the efficacy results for trials 1 and 2.
Table 2: Efficacy Endpoints
|
Trial 1 |
Trial 2 |
|||
|
Efinaconazole Topical Solution |
Vehicle |
Efinaconazole Topical Solution |
Vehicle |
|
|
N = 656 |
N = 214 |
N = 580 |
N = 201 |
|
|
Complete |
117 |
7 |
88 |
11 |
|
Curea |
17.8% |
3.3% |
15.2% |
5.5% |
|
Complete or |
173 |
15 |
136 |
15 |
|
Almost Complete Cureb |
26.4% |
7.0% |
23.4% |
7.5% |
|
Mycologic Curec |
362 |
36 |
310 |
34 |
|
55.2% |
16.8% |
53.4% |
16.9% |
|
a Complete cure defined as 0% clinical involvement of the target toenail plus negative KOH and negative culture.
b Complete or almost complete cure defined as ≤5% affected target toenail area involved and negative KOH and culture.
c Mycologic cure defined as negative KOH and negative culture.
16 HOW SUPPLIED/STORAGE AND HANDLING
Efinaconazole topical solution, 10% is a clear, colorless to pale yellow solution supplied in a white plastic bottle with an integrated flow-through brush applicator as follows:
- •
- 4 mL (NDC 0574-0155-04)
- •
- 8 mL (NDC 0574-0155-08)
Storage and Handling Conditions:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- •
- Solution is flammable; keep away from heat or flame.
- •
- Protect from freezing.
- •
- Keep out of reach of children.
- •
- Keep bottle tightly closed.
- •
- Store in upright position.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Efinaconazole topical solution is for external use only and is not for oral, ophthalmic, or intravaginal use. It is for use on toenails and immediately adjacent skin only.
- •
- Apply efinaconazole topical solution once daily to clean dry toenails. Wait for at least 10 minutes after showering, bathing, or washing before applying.
- •
- Use efinaconazole topical solution only on the affected toenails, as directed by your healthcare provider.
- •
- Inform a healthcare professional if the area of application shows signs of persistent irritation (for example, redness, itching, swelling).
- •
- The impact of nail polish or other cosmetic nail products on the efficacy of efinaconazole topical solution has not been evaluated.
- •
- Flammable, avoid use near heat or open flame.
Manufactured By
Padagis
Minneapolis, MN 55427
www.padagis.com
1W600 RC J1
Rev 04-22
PATIENT INFORMATION
Efinaconazole (ef״-in-a-kon׳-a-zole)
Topical Solution, 10%
Important information: Efinaconazole topical solution is for use on toenails and surrounding skin only. Do not use efinaconazole topical solution in your mouth, eyes, or vagina.
What is efinaconazole topical solution?
Efinaconazole topical solution is a prescription medicine used to treat fungal infections of the toenails.
It is not known if efinaconazole topical solution is safe and effective for use in children under 6 years of age.
What should I tell my healthcare provider before using efinaconazole topical solution?
Before you use efinaconazole topical solution, tell your healthcare provider about all your medical conditions, including if you:
- •
- are pregnant or plan to become pregnant. It is not known if efinaconazole topical solution can harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if efinaconazole passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use efinaconazole topical solution?
See the “Instructions for Use” for detailed information about the right way to use efinaconazole topical solution.
- •
- Use efinaconazole topical solution exactly as your healthcare provider tells you to use it.
- •
- Apply efinaconazole topical solution to your affected toenails 1 time each day. Wait for at least 10 minutes after showering, bathing, or washing before applying efinaconazole topical solution.
- •
- Efinaconazole topical solution is used for 48 weeks.
- •
- It is not known if the use of nail polish or other cosmetic nail products (such as gel nails or acrylic nails) will affect how efinaconazole topical solution works.
What should I avoid while using efinaconazole topical solution?
- •
- Efinaconazole topical solution is flammable. Avoid heat and flame while applying efinaconazole topical solution to your toenail.
What are the possible side effects of efinaconazole topical solution?
Efinaconazole topical solution may cause irritation at the treated site. The most common side effects include: ingrown toenail, redness, itching, swelling, burning or stinging, blisters, and pain. Tell your healthcare provider if you have any side effects that bother you or that do not go away.
These are not all the possible side effects of efinaconazole topical solution.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store efinaconazole topical solution?
- •
- Store efinaconazole topical solution at room temperature, between 68° to 77°F (20° to
25°C). Do not freeze efinaconazole topical solution. - •
- Keep the bottle tightly closed and store in an upright position.
- •
- Efinaconazole topical solution is flammable. Keep away from heat and flame.
Keep efinaconazole topical solution and all medicines out of reach of children.
General information about the safe and effective use of efinaconazole topical solution
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about efinaconazole topical solution that is written for healthcare professionals. Do not use efinaconazole topical solution for a condition for which it was not prescribed. Do not give efinaconazole topical solution to other people, even if they have the same condition you have. It may harm them.
What are the ingredients in efinaconazole topical solution?
Active ingredients: efinaconazole
Inactive ingredients: alcohol (55.8% v/v), anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water.
Manufactured by: Padagis, Minneapolis, MN 55427 USA
For more information, call 1-866-634-9120.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev. 04/2022
Instructions for Use
Efinaconazole (ef״-in-a-kon׳-a-zole)
Topical Solution, 10%
Important information: Efinaconazole topical solution is for use on toenails and surrounding skin only. Do not use efinaconazole topical solution in your mouth, eyes or vagina.
Read this Instructions for Use that comes with efinaconazole topical solution before you start using it. Talk to your healthcare provider if you have any questions.
How to apply efinaconazole topical solution:
Your toenails should be clean and dry before you apply efinaconazole topical solution. Wait at least 10 minutes after showering, bathing, or washing before applying efinaconazole topical solution.
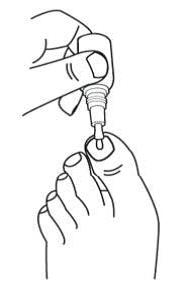
Step 1: Remove the cap from the efinaconazole topical solution bottle.
Step 2: Prepare efinaconazole topical solution for application.
- •
- Hold the efinaconazole topical solution bottle upside down directly over the affected toenail and gently squeeze the bottle to moisten the entire brush with the solution.
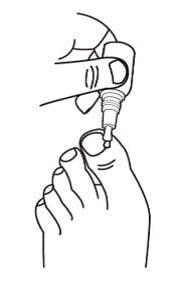
Step 3: Apply efinaconazole topical solution
- •
- While holding the bottle upside down, use the moistened brush to apply efinaconazole topical solution by brushing it gently onto the affected toenail(s). Gently squeeze the efinaconazole topical solution bottle to moisten the brush if needed.
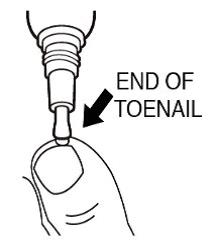
- •
- Gently spread efinaconazole topical solution over the entire toenail around the cuticle, folds of the skin next to the sides of the toenail, and underneath the end of the toenail.
- •
- Do not squeeze bottle while spreading efinaconazole topical solution.
- •
- Do not press or rub the brush firmly against the toenail.
Step 4: For the big toenail, repeat Step 3 to apply efinaconazole topical solution a second time.
Step 5: After applying efinaconazole topical solution, the entire toenail and surrounding skin should be covered with the solution. Let the treated area dry completely before covering it with bedding, socks, or other clothing.
Step 6: Replace the cap tightly on the bottle.
Step 7: Wash your hands with soap and water after applying efinaconazole topical solution.
How should I store efinaconazole topical solution?
- •
- Store efinaconazole topical solution at room temperature, between 68°F to 77°F (20°C to 25°C). Do not freeze efinaconazole topical solution.
- •
- Keep the bottle tightly closed and store in an upright position.
- •
- Efinaconazole topical solution is flammable. Keep away from heat and flame.
Keep efinaconazole topical solution and all medicines out of the reach of children.
This Instructions for Use has been approved by the Food and Drug Administration.
Manufactured by: Padagis, Minneapolis, MN 55427 USA
Rev. 04/2022
| EFINACONAZOLE
efinaconazole solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Padagis US LLC (967694121) |