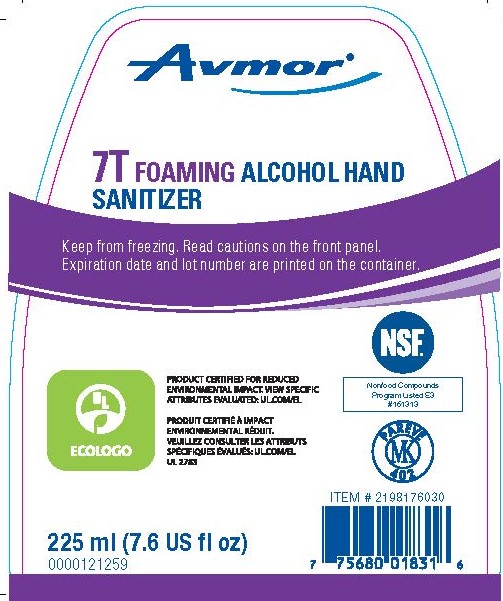
7T FOAMING ALCOHOL HAND SANITIZER- ethyl alcohol liquid
Avmor
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- Kills harmful bacteria/germs.
- For personal hand hygiene to help prevent the spread of bacteria.
Warnings
- For external use only.
- Flammable, keep away from fire or flame.
Directions
- Use as needed.
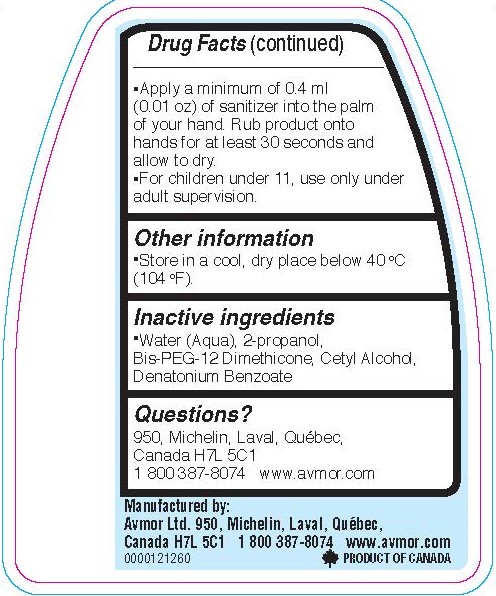
- Apply a minimum of 0.4 ml (0.01 oz) of sanitizer into the palm of your hand. Rub product onto hands for at least 30 seconds and allow to dry.
- For children under 11, use only under adult supervision.
| 7T FOAMING ALCOHOL HAND SANITIZER
ethyl alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Avmor (202107827) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avmor | 202107827 | manufacture(63937-2198) , label(63937-2198) , pack(63937-2198) | |
Revised: 1/2022
Document Id: d58d0489-54b3-461f-e053-2995a90a1f35
Set id: a4e8c29a-5a1b-6d62-e053-2995a90a39e6
Version: 2
Effective Time: 20220114
Avmor