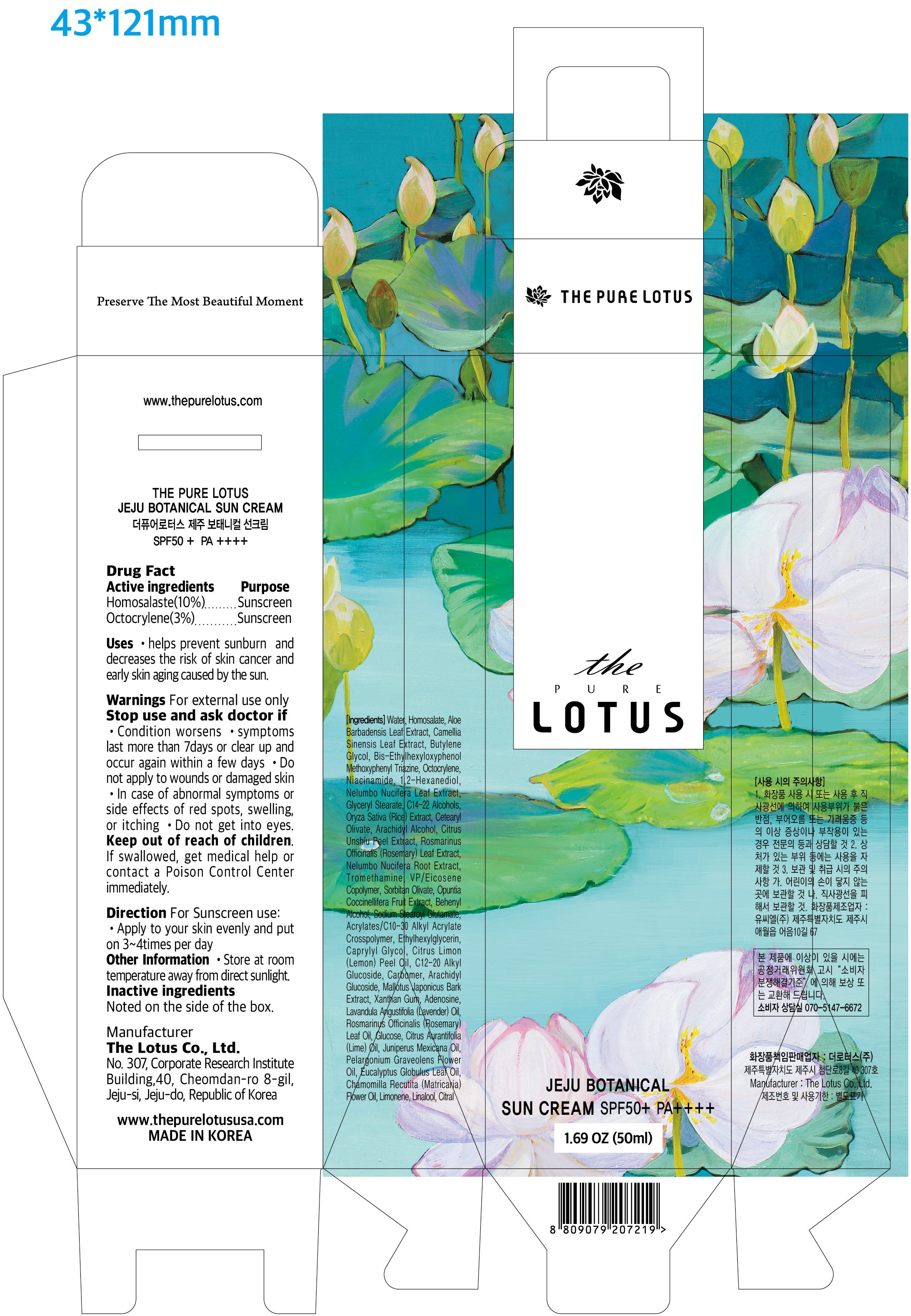
THE PURE LOTUS JEJU BOTANICAL SUNCREAM- homosalate, octocrylene cream
THE LOTUS Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Homosalaste(10%)
Octocrylene(3%)
Uses
- helps prevent sunburn and decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Stop use and ask doctor if
- Condition worsens
- symptoms last more than 7days or clear up and occur again within a few days
Do not use
- Do not apply to wounds or damaged skin
- In case of abnormal symptoms or side effects of red spots, swelling, or itching
- Do not get into eyes.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Direction
For Sunscreen use;
- Apply to your skin evenly and put on 3~4times per day
Other Information
- Store at room temperature away from direct sunlight.
Inactive ingredients
Water, Aloe Barbadensis Leaf Extract, Camellia Sinensis Leaf Extract, Butylene Glycol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Niacinamide, 1,2-Hexanediol, Nelumbo Nucifera Leaf Extract, Glyceryl Stearate, C14-22 Alcohols, Oryza Sativa (Rice) Extract, Cetearyl Olivate, Arachidyl Alcohol, Citrus Unshiu Peel Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Nelumbo Nucifera Root Extract, Tromethamine, VP/Eicosene Copolymer, Sorbitan Olivate, Opuntia Coccinellifera Fruit Extract, Behenyl Alcohol, Sodium Stearoyl Glutamate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Ethylhexylglycerin, Caprylyl Glycol, Citrus Limon (Lemon) Peel Oil, C12-20 Alkyl Glucoside, Carbomer, Arachidyl Glucoside, Mallotus Japonicus Bark Extract, Xanthan Gum, Adenosine, Lavandula Angustifolia (Lavender) Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Glucose, Citrus Aurantifolia (Lime) Oil, Juniperus Mexicana Oil, Pelargonium Graveolens Flower Oil, Eucalyptus Globulus Leaf Oil, Chamomilla Recutita (Matricaria) Flower Oil, Limonene, Linalool, Citral.
Package Label
THE LOTUS Co.,Ltd.
