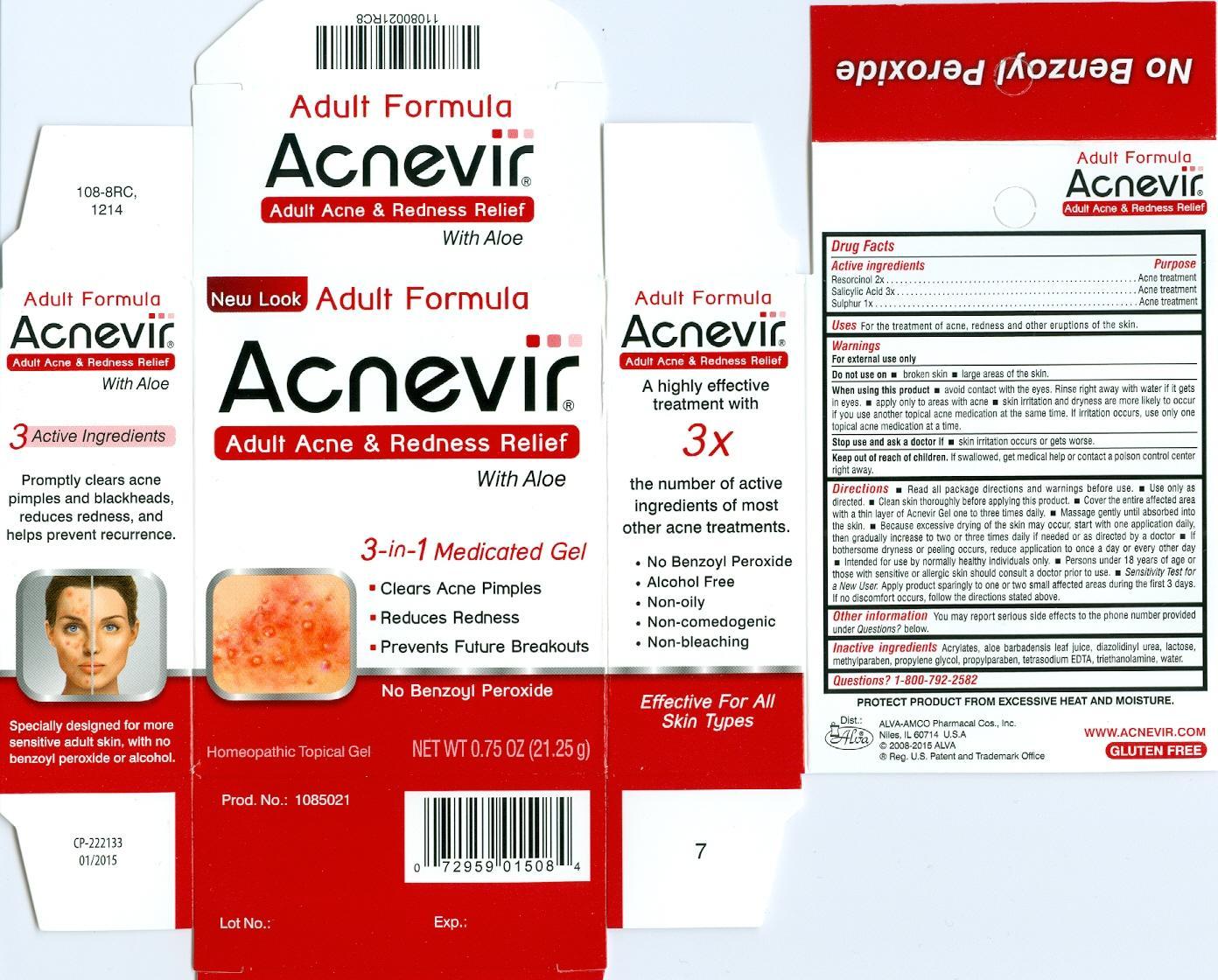
ACNEVIR- resorcinol, salicylic acid, sulphur gel
Denison Pharmaceuticals, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Acnevir Gel
Active ingredients.........................Purpose
Rescorcinol 2x................................Acne treatment
Salicylic Acid 3x..............................Acne treatment
Sulphur 1x......................................Acne treatment
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Warnings
For external use only.
Do not use on
- broken skin
- large areas of the skin
When using this product
- avoid contact with the eyes. Rinse right away with water if it gets in eyes.
- apply only to areas with acne
- skin irritations and dryness are more likely to occur if you use another topical acne medication at the time. If irritation occurs, use only one topical acne medication at a time.
Stop use and ask a doctor if
- skin irritation occurs or gets worse.
Directions
- Read all package directions and warnings before use.
- Only only as directed.
- Clean skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer of Acnevir Gel one to three times a daily.
- Massage gently until absorbed into the skin.
- Because of excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Intended for use by normally healthy individuals only.
- Persons under 18 years of age or those with sensitive of allergic skin should consult a doctor prior to use.
- Sensitivity Test for a New User. Apply product sparingly to one or two affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
Inactive ingredients
Acrylate, aloe barbadensis leaf juice, diazolidinyl urea, lactose, methylparaben, propylene glycol, propylparaben, tetrasodium EDTA, triethanolamine, water
| ACNEVIR
resorcinol, salicylic acid, sulphur gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, LLC (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, LLC | 001207208 | manufacture(0295-1080) | |