avandamet (rosiglitazone maleate and metformin hydrochloride) tablet
[GlaxoSmithKline]
WARNING: CONGESTIVE HEART FAILURE
-
Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients (see WARNINGS, Rosiglitazone maleate). After initiation of AVANDAMET, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of AVANDAMET must be considered.
-
AVANDAMET is not recommended in patients with symptomatic heart failure. Initiation of AVANDAMET in patients with established NYHA Class III or IV heart failure is contraindicated. (See CONTRAINDICATIONS and WARNINGS, Rosiglitazone maleate.)
DESCRIPTION
AVANDAMET (rosiglitazone maleate and metformin HCl) tablets contain 2 oral antihyperglycemic drugs used in the management of type 2 diabetes: Rosiglitazone maleate and metformin hydrochloride.
Rosiglitazone maleate is an oral antidiabetic agent, which acts primarily by increasing insulin sensitivity. Rosiglitazone improves glycemic control while reducing circulating insulin levels. Pharmacologic studies in animal models indicate that rosiglitazone improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Rosiglitazone maleate is not chemically or functionally related to the sulfonylureas, the biguanides, or the α-glucosidase inhibitors.
Chemically, rosiglitazone maleate is (±)-5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]
methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1) with a molecular weight of 473.52 (357.44
free base). The molecule has a single chiral center and is present
as a racemate. Due to rapid interconversion, the enantiomers are functionally
indistinguishable. The molecular formula is C18H19N3O3S•C4H4O4. Rosiglitazone maleate is a white to off-white solid with
a melting point range of 122° to 123°C. The pKa values of rosiglitazone maleate are 6.8 and 6.1. It is readily soluble
in ethanol and a buffered aqueous solution with pH of 2.3; solubility
decreases with increasing pH in the physiological range. The structural
formula of rosiglitazone maleate is:
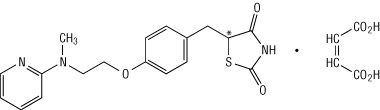
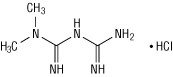
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula of metformin hydrochloride is:
AVANDAMET is available for oral administration as tablets containing rosiglitazone maleate and metformin hydrochloride equivalent to: 2 mg rosiglitazone with 500 mg metformin hydrochloride (2 mg/500 mg), 4 mg rosiglitazone with 500 mg metformin hydrochloride (4 mg/500 mg), 2 mg rosiglitazone with 1,000 mg metformin hydrochloride (2 mg/1,000 mg), and 4 mg rosiglitazone with 1,000 mg metformin hydrochloride (4 mg/1,000 mg). In addition, each tablet contains the following inactive ingredients: Hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone 29-32, sodium starch glycolate, titanium dioxide, and 1 or more of the following: Red and yellow iron oxides.
CLINICAL PHARMACOLOGY
Mechanism of Action
AVANDAMET
AVANDAMET combines 2 antidiabetic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: Rosiglitazone maleate, a member of the thiazolidinedione class, and metformin hydrochloride, a member of the biguanide class. Thiazolidinediones are insulin sensitizing agents that act primarily by enhancing peripheral glucose utilization, whereas biguanides act primarily by decreasing endogenous hepatic glucose production.
Rosiglitazone maleate
Rosiglitazone, a member of the thiazolidinedione class of antidiabetic agents, improves glycemic control by improving insulin sensitivity while reducing circulating insulin levels. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator–activated receptor-gamma (PPARγ). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism.
Insulin resistance is a common feature characterizing the pathogenesis of type 2 diabetes. The antidiabetic activity of rosiglitazone has been demonstrated in animal models of type 2 diabetes in which hyperglycemia and/or impaired glucose tolerance is a consequence of insulin resistance in target tissues. Rosiglitazone reduces blood glucose concentrations and reduces hyperinsulinemia in the ob/ob obese mouse, db/db diabetic mouse, and fa/fa fatty Zucker rat.
In animal models, rosiglitazone’s antidiabetic activity was shown to be mediated by increased sensitivity to insulin's action in the liver, muscle, and adipose tissue. The expression of the insulin-regulated glucose transporter GLUT-4 was increased in adipose tissue. Rosiglitazone did not induce hypoglycemia in animal models of type 2 diabetes and/or impaired glucose tolerance.
Metformin hydrochloride
Metformin hydrochloride is an antihyperglycemic agent, which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and increases peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects (except in special circumstances, see PRECAUTIONS) and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
Pharmacokinetics
Absorption
AVANDAMET
In a bioequivalence and dose proportionality study of AVANDAMET 4 mg/500 mg, both the rosiglitazone component and the metformin component were bioequivalent to coadministered 4 mg rosiglitazone maleate tablet and 500 mg metformin hydrochloride tablet under fasted conditions (see Table 1). In this study, dose proportionality of rosiglitazone in the combination formulations of 1 mg/500 mg and 4 mg/500 mg was demonstrated.
|
Pharmacokinetic Parameter |
|||||
|
Regimen |
N |
AUC0-inf (ng.h/mL) |
Cmax (ng/mL) |
Tmax* (h) |
T½ (h) |
|
Rosiglitazone | |||||
|
A |
25 |
1,442 (324) |
242 (70) |
0.95 (0.48-2.47) |
4.26 (1.18) |
|
B |
25 |
1,398 (340) |
254 (69) |
0.57 (0.43-2.58) |
3.95 (0.81) |
|
C |
24 |
349 (91) |
63.0 (15.0) |
0.57 (0.47-1.45) |
3.87 (0.88) |
|
Metformin | |||||
|
A |
25 |
7,116 (2,096) |
1,106 (329) |
2.97 (1.02-4.02) |
3.46 (0.96) |
|
B |
25 |
7,413 (1,838) |
1,135 (253) |
2.50 (1.03-3.98) |
3.36 (0.54) |
|
C |
24 |
6,945 (2,045) |
1,080 (327) |
2.97 (1.00-5.98) |
3.35 (0.59) |
|
* Median and range presented for Tmax Regimen Key: Regimen A = 4 mg/500 mg AVANDAMET Regimen B = 4 mg rosiglitazone maleate tablet + 500 mg metformin hydrochloride tablet Regimen C = 1 mg/500 mg AVANDAMET |
|||||
Administration of AVANDAMET 4 mg/500 mg with food resulted in no change in overall exposure (AUC) for either rosiglitazone or metformin. However, there were decreases in Cmax of both components (22% for rosiglitazone and 15% for metformin, respectively) and a delay in Tmax of both components (1.5 hours for rosiglitazone and 0.5 hours for metformin, respectively). These changes are not likely to be clinically significant. The pharmacokinetics of both the rosiglitazone component and the metformin component of AVANDAMET when taken with food were similar to the pharmacokinetics of rosiglitazone and metformin when administered concomitantly as separate tablets with food.
Absorption
Rosiglitazone maleate
The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range. The elimination half-life is 3 to 4 hours and is independent of dose.
Absorption
Metformin hydrochloride
The absolute bioavailability of a 500 mg metformin hydrochloride tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin hydrochloride tablets of 500 mg to 1,500 mg, and 850 mg to 2,550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination.
Distribution
Rosiglitazone maleate
The mean (CV%) oral volume of distribution (Vss/F) of rosiglitazone is approximately 17.6 (30%) liters, based on a population pharmacokinetic analysis. Rosiglitazone is approximately 99.8% bound to plasma proteins, primarily albumin.
Distribution
Metformin hydrochloride
The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg metformin hydrochloride averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 mcg/mL. During controlled clinical trials, maximum metformin plasma levels did not exceed 5 mcg/mL, even at maximum doses.
Metabolism and Excretion
Rosiglitazone maleate
Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine. The major routes of metabolism were N-demethylation and hydroxylation, followed by conjugation with sulfate and glucuronic acid. All the circulating metabolites are considerably less potent than parent and, therefore, are not expected to contribute to the insulin-sensitizing activity of rosiglitazone. In vitro data demonstrate that rosiglitazone is predominantly metabolized by cytochrome P450 (CYP) isoenzyme 2C8, with CYP2C9 contributing as a minor pathway. Following oral or intravenous administration of [14C]rosiglitazone maleate, approximately 64% and 23% of the dose was eliminated in the urine and in the feces, respectively. The plasma half-life of [14C]related material ranged from 103 to 158 hours.
Metabolism and Excretion
Metformin hydrochloride
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance is approximately 3.5 times greater than creatinine clearance which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Special Populations
Renal Impairment
In subjects with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance (see WARNINGS, also see GLUCOPHAGE® prescribing information, and CLINICAL PHARMACOLOGY, Pharmacokinetics). Since metformin is contraindicated in patients with renal impairment, administration of AVANDAMET is contraindicated in these patients.
Hepatic Impairment
Unbound oral clearance of rosiglitazone was significantly lower in patients with moderate to severe liver disease (Child-Pugh Class B/C) compared to healthy subjects. As a result, unbound Cmax and AUC0-inf were increased 2- and 3-fold, respectively. Elimination half-life for rosiglitazone was about 2 hours longer in patients with liver disease, compared to healthy subjects.
Therapy with AVANDAMET should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X upper limit of normal) at baseline (see PRECAUTIONS, Hepatic Effects).
No pharmacokinetic studies of metformin have been conducted in subjects with hepatic insufficiency.
Geriatric
Results of the population pharmacokinetics analysis (n = 716 <65 years; n = 331 ≥65 years) showed that age does not significantly affect the pharmacokinetics of rosiglitazone. However, limited data from controlled pharmacokinetic studies of metformin hydrochloride in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see GLUCOPHAGE prescribing information and CLINICAL PHARMACOLOGY, Pharmacokinetics). Metformin treatment and therefore treatment with AVANDAMET should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced (see WARNINGS and DOSAGE AND ADMINISTRATION).
Gender
Results of the population pharmacokinetics analysis showed that the mean oral clearance of rosiglitazone in female patients (n = 405) was approximately 6% lower compared to male patients of the same body weight (n = 642). In rosiglitazone and metformin combination studies, efficacy was demonstrated with no gender differences in glycemic response.
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes when analyzed according to gender (males = 19, females = 16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin hydrochloride tablets was comparable in males and females.
Race
Results of a population pharmacokinetic analysis including subjects of white, black, and other ethnic origins indicate that race has no influence on the pharmacokinetics of rosiglitazone.
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin hydrochloride in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (n = 249), blacks (n = 51), and Hispanics (n = 24).
Pediatric
No pharmacokinetic data from studies in pediatric subjects are available for AVANDAMET.
Pharmacokinetic parameters of rosiglitazone in pediatric patients were established using a population pharmacokinetic analysis with sparse data from 96 pediatric patients in a single pediatric clinical trial including 33 males and 63 females with ages ranging from 10 to 17 years (weights ranging from 35 to 178.3 kg). Population mean CL/F and V/F of rosiglitazone were 3.15 L/hr and 13.5 L, respectively. These estimates of CL/F and V/F were consistent with the typical parameter estimates from a prior adult population analysis.
Drug Interactions
Rosiglitazone maleate
Drugs that Inhibit, Induce, or are Metabolized by Cytochrome P450: In vitro drug metabolism studies suggest that rosiglitazone does not inhibit any of the major P450 enzymes at clinically relevant concentrations. In vitro data demonstrate that rosiglitazone is predominantly metabolized by CYP2C8, and to a lesser extent, 2C9.
Gemfibrozil: Concomitant administration of gemfibrozil (600 mg twice daily), an inhibitor of CYP2C8, and rosiglitazone (4 mg once daily) for 7 days increased rosiglitazone AUC by 127%, compared to the administration of rosiglitazone (4 mg once daily) alone. Given the potential for dose-related adverse events with rosiglitazone, a decrease in the dose of rosiglitazone may be needed when gemfibrozil is introduced.
Rifampin: Rifampin administration (600 mg once a day), an inducer of CYP2C8, for 6 days is reported to decrease rosiglitazone AUC by 66%, compared to the administration of rosiglitazone (8 mg) alone (see PRECAUTIONS).1
Rosiglitazone (4 mg twice daily) was shown to have no clinically relevant effect on the pharmacokinetics of nifedipine and oral contraceptives (ethinyl estradiol and norethindrone), which are predominantly metabolized by CYP3A4.
Metformin hydrochloride
Furosemide: A single-dose, metformin-furosemide drug interaction study in healthy subjects demonstrated that pharmacokinetic parameters of both compounds were affected by coadministration. Furosemide increased the metformin plasma and blood Cmax by 22% and blood AUC by 15%, without any significant change in metformin renal clearance. When administered with metformin, the Cmax and AUC of furosemide were 31% and 12% smaller, respectively, than when administered alone, and the terminal half-life was decreased by 32%, without any significant change in furosemide renal clearance. No information is available about the interaction of metformin and furosemide when coadministered chronically.
Nifedipine: A single-dose, metformin-nifedipine drug interaction study in normal healthy volunteers demonstrated that coadministration of nifedipine increased plasma metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount excreted in the urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of metformin. Metformin had minimal effects on nifedipine.
Cationic Drugs: Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Such interaction between metformin and oral cimetidine has been observed in normal healthy volunteers in both single- and multiple-dose, metformin-cimetidine drug interaction studies, with a 60% increase in peak metformin plasma and whole blood concentrations and a 40% increase in plasma and whole blood metformin AUC. There was no change in elimination half-life in the single-dose study. Metformin had no effect on cimetidine pharmacokinetics.
Other: Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid.
In healthy volunteers, the pharmacokinetics of metformin and propranolol and metformin and ibuprofen were not affected when coadministered in single-dose interaction studies.
Metformin is negligibly bound to plasma proteins and is therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid.
CLINICAL STUDIES
Drug-Naїve Patients with Type 2 Diabetes Mellitus
In a 32-week, randomized, double-blind clinical trial, 468 drug-naïve patients with type 2 diabetes mellitus inadequately controlled with diet and exercise alone (mean baseline FPG 198 mg/dL and mean baseline HbA1c 8.8% ) were randomized to AVANDAMET 2 mg/500 mg, rosiglitazone 4 mg, or metformin 500 mg. Doses were increased at 4-week intervals up to a maximum of 8 mg/2,000 mg for AVANDAMET, 8 mg for rosiglitazone, and 2,000 mg for metformin to reach a target mean daily glucose of ≤110 mg/dL. Following the initial dosage level, AVANDAMET, rosiglitazone, and metformin were all administered as twice daily regimens. Statistically significant improvements in FPG and HbA1c were observed in patients treated with AVANDAMET compared to either rosiglitazone or metformin alone (see Table 2). However, when considering the choice of therapy for drug-naïve patients, the risk-benefit of initiating monotherapy or dual therapy should be considered.
|
|
AVANDAMET |
Rosiglitazone |
Metformin |
|
Mean Final Dose |
7.2 mg/1,799 mg |
7.7 mg |
1,847 mg |
|
N |
152 |
155 |
150 |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
201 |
194 |
199 |
|
Change from baseline (mean) |
–74 |
–47 |
–51 |
|
Difference between AVANDAMET and monotherapy (adjusted mean) |
–22* |
–22* |
|
|
% of patients with ≥30 mg/dL decrease from baseline |
86% |
68% |
64% |
|
HbA1c (%) | |||
|
Baseline (mean) |
8.9% |
8.8% |
8.8% |
|
Change from baseline (mean) |
–2.3% |
–1.6% |
–1.8% |
|
Difference between AVANDAMET and monotherapy (adjusted mean) |
–0.6* |
–0.4* |
|
|
% of patients with HbA1c ≥0.7% decrease from baseline |
92% |
79% |
84% |
|
% of Patients with HbA1c <7.0% |
77% |
58% |
57% |
|
* p<0.001 AVANDAMET compared to rosiglitazone or metformin. |
|||
The lipid profiles of AVANDAMET as well as rosiglitazone and metformin monotherapies are shown in Table 3.
|
AVANDAMET N† = 132 |
Rosiglitazone N† = 128 |
Metformin N† = 117 |
|
|
Total Cholesterol (mg/dL) | |||
|
Baseline (mean) |
200.4 |
198.4 |
201.6 |
|
% Change from baseline (mean) |
–2.2% |
5.3% |
–9.0% |
|
LDL (mg/dL) | |||
|
Baseline (mean) |
113.8 |
114.6 |
116.0 |
|
% Change from baseline (mean) |
–0.2% |
4.5% |
–10.7% |
|
HDL (mg/dL) | |||
|
Baseline (mean) |
42.6 |
42.8 |
42.9 |
|
% Change from baseline (mean) |
5.8% |
3.1% |
0.0% |
|
Triglycerides (mg/dL) | |||
|
Baseline (mean) |
180.3 |
166.6 |
175.7 |
|
% Change from baseline (mean) |
–18.7% |
–4.8% |
–15.4% |
|
* Data presented as geometric means throughout table. † N = number of subjects with a baseline and end of treatment value. |
|||
Patients screened in the double-blind clinical trial described above with HbA1c >11% or FPG >270 mg/dL were not eligible for blinded treatment but were treated with open-label AVANDAMET (4 mg/1,000 mg up to a maximum dose of 8 mg/2,000 mg). Treatment with AVANDAMET reduced mean HbA1c from a baseline of 11.8% to 7.8% and mean FPG from a baseline of 305 mg/dL to 166 mg/dL. Given the lack of direct comparators in this evaluation, determination of the exact contribution of rosiglitazone and metformin as well as diet and exercise, to the observed improvement in glycemic control is not possible.
AVANDAMET Therapy in Patients with Type 2 Diabetes Mellitus Treated with Metformin Hydrochloride
AVANDAMET was not studied in patients previously treated with metformin monotherapy; however, the combination of rosiglitazone maleate and metformin hydrochloride was compared to rosiglitazone and metformin monotherapies in clinical trials. Bioequivalence between AVANDAMET and coadministered rosiglitazone maleate tablets and metformin hydrochloride tablets has been demonstrated (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
The pattern of LDL, HDL, and total cholesterol changes following therapy with rosiglitazone in combination with metformin was generally similar to those seen with rosiglitazone monotherapy, and a small decrease in mean triglycerides was observed with the combination therapy.
A total of 670 patients with type 2 diabetes participated in two 26-week, randomized, double-blind, placebo/active-controlled studies designed to assess the efficacy of rosiglitazone in combination with metformin. Rosiglitazone maleate, administered in either once-daily or twice-daily dosing regimens, was added to the therapy of patients who were inadequately controlled on 2.5 grams/day of metformin hydrochloride.
In one study, patients inadequately controlled on 2.5 grams/day of metformin hydrochloride (mean baseline FPG 216 mg/dL and mean baseline HbA1c 8.8%) were randomized to receive rosiglitazone 4 mg once daily, rosiglitazone 8 mg once daily, or placebo in addition to metformin. A statistically significant improvement in FPG and HbA1c was observed in patients treated with the combinations of metformin and rosiglitazone 4 mg once daily and rosiglitazone 8 mg once daily, versus patients continued on metformin alone (see Table 4).
|
|
Metformin |
Rosiglitazone 4 mg once daily + metformin |
Rosiglitazone 8 mg once daily + metformin |
|
N |
113 |
116 |
110 |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
214 |
215 |
220 |
|
Change from baseline (mean) |
6 |
–33 |
–48 |
|
Difference from metformin alone (adjusted mean) |
–40* |
–53* |
|
|
% of patients with ≥30 mg/dL decrease from baseline |
20% |
45% |
61% |
|
HbA1c (%) | |||
|
Baseline (mean) |
8.6 |
8.9 |
8.9 |
|
Change from baseline (mean) |
0.5 |
–0.6 |
–0.8 |
|
Difference from metformin alone (adjusted mean) |
–1.0* |
–1.2* |
|
|
% of patients with HbA1c ≥0.7% decrease from baseline |
11% |
45% |
52% |
|
* p<0.0001 compared to metformin. |
|||
In a second 26-week study, patients with type 2 diabetes inadequately controlled on 2.5 grams/day of metformin hydrochloride who were randomized to receive the combination of rosiglitazone 4 mg twice daily and metformin (N = 105) showed a statistically significant improvement in glycemic control with a mean treatment effect for FPG of –56 mg/dL and a mean treatment effect for HbA1c of –0.8% over metformin alone. The combination of metformin and rosiglitazone resulted in lower levels of FPG and HbA1c than either agent alone.
AVANDAMET Combination with Insulin: After an 8-week single-blind AVANDAMET run-in (rosiglitazone 8 mg/metformin 2,000 mg daily), 324 patients with type 2 diabetes mellitus with fasting plasma glucose (FPG) ≥126 mg/dL were randomized to receive AVANDAMET 8 mg/2,000 mg plus insulin (insulin add-on therapy) or placebo plus insulin (switch to insulin monotherapy) in a 24-week, double-blind, multicenter study. Most of the patients had been treated with metformin therapy (∼20% monotherapy and ∼70% combination therapy with a sulfonylurea) before entering the AVANDAMET run-in. Patients with congestive heart failure or who developed edema or whose edema worsened on AVANDAMET therapy during the run-in were not eligible for randomization. Premixed insulin was initiated at 12 units administered twice daily and could be adjusted at a minimum of every 3 to 5 days to achieve target capillary blood glucose values (pre-breakfast and pre-evening meals FPG ≤117.0 mg/dL).
|
|
AVANDAMET + Insulin |
Insulin Monotherapy |
|
N |
161 |
157 |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
196 |
195 |
|
Mean change from baseline |
–61 |
–34 |
|
Difference from insulin monotherapy |
–26* | |
|
% of patients with ≥30 mg/dL decrease from baseline |
71% |
48% |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.7 |
8.8 |
|
Mean change from baseline |
–2.0 |
–1.3 |
|
% of patients with HbA1c ≥0.7% decrease from baseline |
84% |
72% |
|
Difference from insulin monotherapy |
–0.7* | |
|
% of patients with HbA1c <7% |
70% |
34% |
|
* Adjusted mean, p<0.0001 compared to insulin monotherapy. |
||
Patients who had insulin added to maximal AVANDAMET therapy had significantly greater reductions in FPG and HbA1c compared to patients who were switched to insulin monotherapy (see Table 5). At Week 24, the mean final total daily insulin dose was significantly lower in the AVANDAMET plus insulin group compared to the insulin monotherapy group (33 U versus 59 U; mean adjusted treatment difference of 25 U, p<0.0001).
INDICATIONS AND USAGE
AVANDAMET is indicated as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus when treatment with dual rosiglitazone and metformin therapy is appropriate.
Management of type 2 diabetes mellitus should include diet control. Caloric restriction, weight loss, and exercise are essential for the proper treatment of the diabetic patient because they help improve insulin sensitivity. This is important not only in the primary treatment of type 2 diabetes but also in maintaining the efficacy of drug therapy. Prior to initiation or escalation of oral antidiabetic therapy in patients with type 2 diabetes mellitus, secondary causes of poor glycemic control, e.g., infection, should be investigated and treated.
CONTRAINDICATIONS
Initiation of AVANDAMET in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated (see BOXED WARNING).
AVANDAMET is contraindicated in patients with renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males], ≥1.4 mg/dL [females], or abnormal creatinine clearance), which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia (see WARNINGS and PRECAUTIONS).
AVANDAMET is contraindicated in patients with known hypersensitivity to rosiglitazone maleate or metformin hydrochloride.
AVANDAMET is contraindicated in patients with acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma. Diabetic ketoacidosis should be treated with insulin.
AVANDAMET should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function (see also PRECAUTIONS).
WARNINGS
Metformin hydrochloride
Lactic Acidosis
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with AVANDAMET; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 mcg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1,000 patient years of exposure, with approximately 0.015 fatal cases/1,000 patient years of exposure). Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking AVANDAMET and by use of the minimum effective dose of AVANDAMET. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Treatment with AVANDAMET should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, AVANDAMET should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, AVANDAMET should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking AVANDAMET, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, AVANDAMET should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see also PRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see also PRECAUTIONS). AVANDAMET should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose and, if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of AVANDAMET, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking AVANDAMET do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity or technical problems in sample handling (see also PRECAUTIONS).
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking AVANDAMET, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery (see also CONTRAINDICATIONS and PRECAUTIONS).
Rosiglitazone maleate
Cardiac Failure and Other Cardiac Effects: Rosiglitazone, like other thiazolidinediones, alone or in combination with other antidiabetic agents, can cause fluid retention, which may exacerbate or lead to heart failure. Patients should be observed for signs and symptoms of heart failure. If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of rosiglitazone must be considered (see BOXED WARNING). AVANDAMET should be discontinued if any deterioration in cardiac status occurs.
Patients with congestive heart failure (CHF) NYHA Class I and II treated with rosiglitazone have an increased risk of cardiovascular events. A 52-week, double-blind, placebo-controlled echocardiographic study was conducted in 224 patients with type 2 diabetes mellitus and NYHA Class I or II CHF (ejection fraction≤45%) on background antidiabetic and CHF therapy. An independent committee conducted a blinded evaluation of fluid-related events (including congestive heart failure) and cardiovascular hospitalizations according to predefined criteria (adjudication). Separate from the adjudication, other cardiovascular adverse events were reported by investigators. Although no treatment difference in change from baseline of ejection fractions was observed, more cardiovascular adverse events were observed with rosiglitazone treatment compared to placebo during the 52-week study. (See Table 6.)
|
Placebo |
Rosiglitazone |
|
|
Events |
N = 114 |
N = 110 |
|
n (%) |
n (%) |
|
|
Adjudicated | ||
|
Cardiovascular deaths |
4 (4) |
5 (5) |
|
CHF worsening |
4 (4) |
7 (6) |
|
4 (4) |
5 (5) |
|
0 (0) |
2 (2) |
|
New or worsening edema |
10 (9) |
28 (25) |
|
New or worsening dyspnea |
19 (17) |
29 (26) |
|
Increases in CHF medication |
20 (18) |
36 (33) |
|
Cardiovascular hospitalization* |
15 (13) |
21 (19) |
|
Investigator-reported, Non-adjudicated | ||
|
Ischemic adverse events |
5 (4) |
10 (9) |
|
2 (2) |
5 (5) |
|
3 (3) |
6 (5) |
|
* Includes hospitalization for any cardiovascular reason. |
||
Initiation of AVANDAMET in patients with established NYHA Class III or IV heart failure is contraindicated. AVANDAMET is not recommended in patients with symptomatic heart failure. (See BOXED WARNING.)
Patients with NYHA Class III and IV cardiac status were not studied during the clinical trials. AVANDAMET is not recommended in patients with NYHA Class III and IV cardiac status.
In combination with insulin, thiazolidinediones may increase the risk of other cardiovascular adverse events. In three 26-week trials in patients with type 2 diabetes, 216 received 4 mg of rosiglitazone plus insulin, 322 received 8 mg of rosiglitazone plus insulin, and 338 received insulin alone. These trials included patients with long-standing diabetes and a high prevalence of pre-existing medical conditions, including peripheral neuropathy, retinopathy, ischemic heart disease, vascular disease, and congestive heart failure. In these clinical studies, an increased incidence of edema, cardiac failure, and other cardiovascular adverse events was seen in patients on rosiglitazone and insulin combination therapy compared to insulin and placebo. Patients who experienced cardiovascular events were on average older and had a longer duration of diabetes. These cardiovascular events were noted at both the 4 mg and 8 mg daily doses of rosiglitazone. In this population, however, it was not possible to determine specific risk factors that could be used to identify all patients at risk of heart failure and other cardiovascular events on combination therapy. Three of 10 patients who developed cardiac failure on combination therapy during the double-blind part of the fixed-dose studies had no known prior evidence of congestive heart failure, or pre-existing cardiac condition.
In a double-blind study in type 2 diabetes patients with chronic renal failure (112 received 4 mg or 8 mg of rosiglitazone plus insulin and 108 received insulin alone), there was no difference in cardiovascular adverse events with rosiglitazone in combination with insulin compared to insulin alone.
Patients treated with combination AVANDAMET and insulin should be monitored for cardiovascular adverse events. The combination therapy should be discontinued in patients who do not respond as manifested by a reduction in HbA1c or insulin dose after 4 to 5 months of therapy or who develop any significant adverse events. (See ADVERSE REACTIONS.)
PRECAUTIONS
Metformin hydrochloride
Monitoring of renal function: Metformin is known to be substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of impairment of renal function. Thus, patients with serum creatinine levels above the upper limit of normal for their age should not receive AVANDAMET. In patients with advanced age, AVANDAMET should be carefully titrated to establish the minimum dose for adequate glycemic effect, because aging is associated with reduced renal function. In elderly patients, particularly those ≥80 years of age, renal function should be monitored regularly and, generally, AVANDAMET should not be titrated to the maximum dose of the metformin component, i.e., 2,000 mg (see WARNINGS and DOSAGE AND ADMINISTRATION).
Before initiation of therapy with AVANDAMET and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal dysfunction is anticipated, renal function should be assessed more frequently and AVANDAMET discontinued if evidence of renal impairment is present.
Use of concomitant medications that may affect renal function or metformin disposition: Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or may interfere with the disposition of metformin, such as cationic drugs that are eliminated by renal tubular secretion (see PRECAUTIONS, Drug Interactions), should be used with caution.
Radiologic studies involving the use of intravascular iodinated contrast materials (for example, intravenous urogram, intravenous cholangiography, angiography, and computed tomography [CT] scans with contrast materials): Intravascular contrast studies with iodinated materials can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin (see CONTRAINDICATIONS). Therefore, in patients in whom any such study is planned, AVANDAMET should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal.
Hypoxic states: Cardiovascular collapse (shock) from whatever cause, acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients receiving AVANDAMET, the drug should be promptly discontinued.
Surgical procedures: Use of AVANDAMET should be temporarily suspended for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient's oral intake has resumed and renal function has been evaluated as normal.
Alcohol intake: Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute or chronic, while receiving AVANDAMET.
Impaired hepatic function: Since impaired hepatic function has been associated with some cases of lactic acidosis, AVANDAMET should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
Vitamin B12 levels: In controlled clinical trials of metformin hydrochloride of 29 weeks’ duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on AVANDAMET and any apparent abnormalities should be appropriately investigated and managed (see PRECAUTIONS, Laboratory Tests). Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurements at 2- to 3-year intervals may be useful.
Change in clinical status of previously controlled diabetic: A patient with type 2 diabetes previously well-controlled on AVANDAMET who develops laboratory abnormalities or clinical illness (especially vague and poorly defined illness) should be evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If acidosis of either form occurs, AVANDAMET must be stopped immediately and other appropriate corrective measures initiated (see also WARNINGS).
Hypoglycemia: Hypoglycemia does not occur in patients receiving metformin hydrochloride alone under usual circumstances of use but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with hypoglycemic agents (such as sulfonylureas or insulin) or ethanol. Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly and in people who are takingβ-adrenergic blocking drugs.
Loss of control of blood glucose: When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of glycemic control may occur. At such times, it may be necessary to withhold AVANDAMET and temporarily administer insulin. AVANDAMET may be reinstituted after the acute episode is resolved.
Rosiglitazone maleate
General: Due to its mechanism of action, rosiglitazone is active only in the presence of endogenous insulin. Therefore, AVANDAMET should not be used in patients with type 1 diabetes.
Hypoglycemia: Patients receiving rosiglitazone in combination with other hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of the concomitant agent may be necessary.
Edema: AVANDAMET should be used with caution in patients with edema. In a clinical study in healthy volunteers who received rosiglitazone 8 mg once daily for 8 weeks, there was a statistically significant increase in median plasma volume compared to placebo. Since thiazolidinediones, including rosiglitazone, can cause fluid retention, which can exacerbate or lead to congestive heart failure, AVANDAMET should be used with caution in patients at risk for heart failure. Patients should be monitored for signs and symptoms of heart failure (see BOXED WARNING, WARNINGS, Rosiglitazone maleate, and PRECAUTIONS).
In controlled clinical trials of patients with type 2 diabetes, mild to moderate edema was reported in patients treated with rosiglitazone maleate, and may be dose related. Patients with ongoing edema are more likely to have adverse events associated with edema if started on combination therapy with insulin and rosiglitazone (see ADVERSE REACTIONS).
Macular Edema: Macular edema has been reported in postmarketing experience in some diabetic patients who were taking rosiglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but some patients appear to have been diagnosed on routine ophthalmologic examination. Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of their thiazolidinedione. Patients with diabetes should have regular eye exams by an ophthalmologist, per the Standards of Care of the American Diabetes Association. Additionally, any diabetic who reports any kind of visual symptom should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings. (See ADVERSE REACTIONS, Postmarketing Experience.)
Fractures: In a 4- to 6-year comparative study (ADOPT) of glycemic control with monotherapy in drug-naïve patients recently diagnosed with type 2 diabetes mellitus, an increased incidence of bone fracture was noted in female patients taking rosiglitazone. Over the 4- to 6-year period, the incidence of bone fracture in females was 9.3% (60/645) for rosiglitazone versus 3.5% (21/605) for glyburide and 5.1% (30/590) for metformin. This increased incidence was noted after the first year of treatment and persisted during the course of the study. The majority of the fractures in the women who received rosiglitazone occurred in the upper arm, hand, and foot. These sites of fracture are different from those usually associated with postmenopausal osteoporosis (e.g., hip or spine). No increase in fracture rates was observed in men treated with rosiglitazone. The risk of fracture should be considered in the care of patients, especially female patients, treated with rosiglitazone, and attention given to assessing and maintaining bone health according to current standards of care.
Weight Gain: Dose-related weight gain was seen with rosiglitazone alone and rosiglitazone together with other hypoglycemic agents (see Table 7). No overall change in median weight was observed with AVANDAMET in drug-naïve patients. The mechanism of weight gain with rosiglitazone is unclear but probably involves a combination of fluid retention and fat accumulation.
|
[Median (25th, 75th, Percentile)] |
||||||
|
Monotherapy |
||||||
|
Duration |
Control Group |
Rosiglitazone 4 mg |
Rosiglitazone 8 mg |
|||
|
26 weeks |
Placebo |
–0.9 (–2.8, 0.9) n = 210 |
1.0 (0.9, 3.6) n = 436 |
3.1 (1.1, 5.8) n = 439 |
||
|
52 weeks |
Sulfonylurea |
2.0 (0, 4.0) n = 173 |
2.0 (–0.6, 4.0) n = 150 |
2.6 (0, 5.3) n = 157 |
||
|
Combination Therapy |
||||||
|
Rosiglitazone plus Control Therapy |
||||||
|
Duration |
Control Group |
Rosiglitazone 4 mg |
Rosiglitazone 8 mg |
|||
|
24-26 weeks |
Sulfonylurea |
0 (–1.0, 1.3) n = 1,155 |
2.2 (0.5, 4.0) n = 613 |
3.5 (1.4, 5.9) n = 841 |
||
|
26 weeks |
Metformin |
–1.4 (–3.2, 0.2) n = 175 |
0.8 (–1.0, 2.6) n = 100 |
2.1 (0, 4.3) n = 184 |
||
|
26 weeks |
Insulin |
0.9 (–0.5, 2.7) n = 162 |
4.1 (1.4, 6.3) n = 164 |
5.4 (3.4, 7.3) n = 150 |
||
|
AVANDAMET in Drug Naïve Patients |
||||||
|
Duration |
Control Groups |
AVANDAMET |
||||
|
32 weeks |
Metformin |
–2.2 (–5.5, –0.5) n = 123 |
0.05 kg (–3.45, 3.0) n = 136 |
|||
|
Rosiglitazone |
1.7 (–1.2, 4.5) n = 136 |
|||||
|
AVANDAMET plus Insulin |
||||||
|
Duration |
Control Group |
AVANDAMET plus Insulin |
||||
|
24 weeks |
Insulin |
2.6 kg (0.3, 4.8) n = 145 |
3.3 kg (1.5, 6.0) n = 147 |
|||
In postmarketing experience with rosiglitazone alone or in combination with other hypoglycemic agents, there have been rare reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure (see BOXED WARNING).
Hematologic: Across all controlled clinical studies in adults, decreases in hemoglobin and hematocrit (mean decreases in individual studies of approximately ≤1.0 gram/dL and≤3.3%, respectively) were observed for rosiglitazone maleate alone and in combination with other hypoglycemic agents. The changes occurred primarily during the first 3 months following initiation of rosiglitazone therapy or following an increase in rosiglitazone dose. The decrease in hemoglobin was seen more frequently in combination rosiglitazone and metformin therapy than in rosiglitazone therapy alone. Vitamin B12 deficiency may contribute to the observed reductions in hemoglobin (see PRECAUTIONS, Metformin hydrochloride, Vitamin B12 levels). White blood cell counts also decreased slightly in adult patients treated with rosiglitazone. Small decreases in hemoglobin and hematocrit have also been reported in pediatric patients treated with rosiglitazone. The observed changes may be related to the increased plasma volume observed with treatment with rosiglitazone and may be dose related (see ADVERSE REACTIONS, Laboratory Abnormalities).
Ovulation: Therapy with rosiglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking AVANDAMET (see PRECAUTIONS, Pregnancy, Pregnancy Category C). Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical studies so the frequency of this occurrence is not known.
Although hormonal imbalance has been seen in preclinical studies (see PRECAUTIONS Carcinogenesis, Mutagenesis, Impairment of Fertility), the clinical significance of this finding is not known. If unexpected menstrual dysfunction occurs, the benefits of continued therapy with AVANDAMET should be reviewed.
Hepatic Effects: Another drug of the thiazolidinedione class, troglitazone, was associated with idiosyncratic hepatotoxicity, and very rare cases of liver failure, liver transplants, and death were reported during clinical use. In pre-approval controlled clinical trials in patients with type 2 diabetes, troglitazone was more frequently associated with clinically significant elevations in liver enzymes (ALT >3X upper limit of normal) compared to placebo. Very rare cases of reversible jaundice were also reported.
In pre-approval clinical studies in 4,598 patients treated with rosiglitazone maleate, encompassing approximately 3,600 patient years of exposure, there was no signal of drug-induced hepatotoxicity or elevation of ALT levels. In the pre-approval controlled trials, 0.2% of patients treated with rosiglitazone had elevations in ALT >3X the upper limit of normal compared to 0.2% on placebo and 0.5% on active comparators. The ALT elevations in patients treated with rosiglitazone were reversible and were not clearly causally related to therapy with rosiglitazone.
In postmarketing experience with rosiglitazone maleate, reports of hepatitis and of hepatic enzyme elevations to 3 or more times the upper limit of normal have been received. Very rarely, these reports have involved hepatic failure with and without fatal outcome, although causality has not been established. Rosiglitazone is structurally related to troglitazone, a thiazolidinedione no longer marketed in the United States, which was associated with idiosyncratic hepatotoxicity and rare cases of liver failure, liver transplants, and death during clinical use. Pending the availability of the results of additional large, long-term controlled clinical trials and additional postmarketing safety data, it is recommended that patients treated with AVANDAMET undergo periodic monitoring of liver enzymes.
Liver enzymes should be checked prior to the initiation of therapy with AVANDAMET in all patients and periodically thereafter per the clinical judgement of the healthcare professional. Therapy with AVANDAMET should not be initiated in patients with increased baseline liver enzyme levels (ALT >2.5X upper limit of normal). Patients with mildly elevated liver enzymes (ALT levels ≤2.5X upper limit of normal) at baseline or during therapy with AVANDAMET should be evaluated to determine the cause of the liver enzyme elevation. Initiation of, or continuation of, therapy with AVANDAMET in patients with mild liver enzyme elevations should proceed with caution and include close clinical follow-up, including more frequent liver enzyme monitoring, to determine if the liver enzyme elevations resolve or worsen. If at any time ALT levels increase to >3X the upper limit of normal in patients on therapy with AVANDAMET, liver enzyme levels should be rechecked as soon as possible. If ALT levels remain >3X the upper limit of normal, therapy with AVANDAMET should be discontinued.
If any patient develops symptoms suggesting hepatic dysfunction, which may include unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, and/or dark urine, liver enzymes should be checked. If jaundice is observed, drug therapy should be discontinued.
In addition, if the presence of hepatic disease or hepatic dysfunction of sufficient magnitude to predispose to lactic acidosis is confirmed, therapy with AVANDAMET should be discontinued.
There are no data available from clinical trials to evaluate the safety of AVANDAMET in patients who experienced liver abnormalities, hepatic dysfunction, or jaundice while on troglitazone. AVANDAMET should not be used in patients who experienced jaundice while taking troglitazone.
Laboratory Tests
Periodic fasting blood glucose and HbA1c measurements should be performed to monitor therapeutic response.
Liver enzyme monitoring is recommended prior to initiation of therapy with AVANDAMET in all patients and periodically thereafter (see PRECAUTIONS, Hepatic Effects and ADVERSE REACTIONS, Laboratory Abnormalities, Serum Transaminase Levels).
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) and renal function (serum creatinine) should be performed, at least on an annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is suspected, vitamin B12 deficiency should be excluded.
Information for Patients
Patients should be informed of the potential risks and advantages of AVANDAMET and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, weight loss, and a regular exercise program because these methods help improve insulin sensitivity. The importance of regular testing of blood glucose, glycosylated hemoglobin (HbA1c), renal function, and hematologic parameters should be emphasized. Patients should be advised that AVANDAMET can begin to take effect 1 to 2 weeks after initiation, however it can take 2 to 3 months to see the full effect of glycemic improvement.
The risks of lactic acidosis, its symptoms, and conditions that predispose to its development, as noted in the WARNINGS and PRECAUTIONS sections, should be explained to patients. Patients should be advised to discontinue AVANDAMET immediately and to promptly notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, or other nonspecific symptoms occur. Once a patient is stabilized on any dose level of AVANDAMET, gastrointestinal symptoms, which are common during initiation of metformin therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Patients should be counseled against excessive alcohol intake, either acute or chronic, while receiving AVANDAMET.
Patients should be informed that blood will be drawn to check their liver function prior to the start of therapy and periodically thereafter per the clinical judgement of the healthcare professional. Patients with unexplained symptoms of nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine should immediately report these symptoms to their physician.
Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on AVANDAMET should immediately report these symptoms to their physician.
Therapy with AVANDAMET, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking AVANDAMET (see PRECAUTIONS, Pregnancy, Pregnancy Category C). Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical studies so the frequency of this occurrence is not known.
Drug Interactions
An inhibitor of CYP2C8 (such as gemfibrozil) may increase the AUC of rosiglitazone and an inducer of CYP2C8 (such as rifampin) may decrease the AUC of rosiglitazone. Therefore, if an inhibitor or an inducer of CYP2C8 is started or stopped during treatment with rosiglitazone, changes in diabetes treatment may be needed based upon clinical response.
Although drug interactions with cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin) remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of AVANDAMET and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system.
When drugs that produce hyperglycemia which may lead to loss of glycemic control are administered to a patient receiving AVANDAMET, the patient should be closely observed to maintain adequate glycemic control. (See CLINICAL PHARMACOLOGY, Drug Interactions.)
Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with the combined products in AVANDAMET. The following data are based on findings in studies performed with rosiglitazone or metformin individually.
Rosiglitazone maleate
A 2-year carcinogenicity study was conducted in Charles River CD-1 mice at doses of 0.4, 1.5, and 6 mg/kg/day in the diet (highest dose equivalent to approximately 12 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). Sprague-Dawley rats were dosed for 2 years by oral gavage at doses of 0.05, 0.3, and 2 mg/kg/day (highest dose equivalent to approximately 10 and 20 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET for male and female rats, respectively).
Rosiglitazone was not carcinogenic in the mouse. There was an increase in incidence of adipose hyperplasia in the mouse at doses ≥1.5 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). In rats, there was a significant increase in the incidence of benign adipose tissue tumors (lipomas) at doses ≥0.3 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). These proliferative changes in both species are considered due to the persistent pharmacological overstimulation of adipose tissue.
Rosiglitazone was not mutagenic or clastogenic in the in vitro bacterial assays for gene mutation, the in vitro chromosome aberration test in human lymphocytes, the in vivo mouse micronucleus test, and the in vivo/in vitro rat UDS assay. There was a small (about 2-fold) increase in mutation in the in vitro mouse lymphoma assay in the presence of metabolic activation.
Rosiglitazone had no effects on mating or fertility of male rats given up to 40 mg/kg/day (approximately 116 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). Rosiglitazone altered estrous cyclicity (2 mg/kg/day) and reduced fertility (40 mg/kg/day) of female rats in association with lower plasma levels of progesterone and estradiol (approximately 20 and 200 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). No such effects were noted at 0.2 mg/kg/day (approximately 3 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). In juvenile rats dosed from 27 days of age through to sexual maturity (at up to 40 mg/kg/day), there was no effect on male reproductive performance, or on estrous cyclicity, mating performance or pregnancy incidence in females (approximately 68 times human AUC at the maximum recommended daily dose of rosiglitazone). In monkeys, rosiglitazone (0.6 and 4.6 mg/kg/day; approximately 3 and 15 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively) diminished the follicular phase rise in serum estradiol with consequential reduction in the luteinizing hormone surge, lower luteal phase progesterone levels, and amenorrhea. The mechanism for these effects appears to be direct inhibition of ovarian steroidogenesis.
Metformin hydrochloride
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1,500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2,000 mg of the metformin component of AVANDAMET based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administrated at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose of the metformin component of AVANDAMET based on body surface area comparisons.
Animal Toxicology
Heart weights were increased in mice (3 mg/kg/day), rats (5 mg/kg/day), and dogs (2 mg/kg/day) with rosiglitazone treatments (approximately 5, 22, and 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). Effects in juvenile rats were consistent with those seen in adults. Morphometric measurement indicated that there was hypertrophy in cardiac ventricular tissues, which may be due to increased heart work as a result of plasma volume expansion.
Pregnancy
Pregnancy Category C. All pregnancies have a background risk of birth defects, loss, or other adverse outcome regardless of drug exposure. This background risk is increased in pregnancies complicated by hyperglycemia and may be decreased with good metabolic control. It is essential for patients with diabetes or history of gestational diabetes to maintain good metabolic control before conception and throughout pregnancy. Careful monitoring of glucose control is essential in such patients. Most experts recommend that insulin monotherapy be used during pregnancy to maintain blood glucose levels as close to normal as possible. AVANDAMET should not be used during pregnancy.
Human Data: There are no adequate and well-controlled studies in pregnant women with AVANDAMET or its individual components.
Rosiglitazone maleate: Rosiglitazone has been reported to cross the human placenta and be detectable in fetal tissue. The clinical significance of these findings is unknown.
Animal Studies: No animal studies have been conducted with the combined products in AVANDAMET. The following data are based on findings in studies performed with rosiglitazone or metformin individually.
Rosiglitazone maleate
There was no effect on implantation or the embryo with rosiglitazone treatment during early pregnancy in rats, but treatment during mid-late gestation was associated with fetal death and growth retardation in both rats and rabbits. Teratogenicity was not observed at doses up to 3 mg/kg in rats and 100 mg/kg in rabbits (approximately 20 and 75 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). Rosiglitazone caused placental pathology in rats (3 mg/kg/day). Treatment of rats during gestation through lactation reduced litter size, neonatal viability, and postnatal growth, with growth retardation reversible after puberty. For effects on the placenta, embryo/fetus, and offspring, the no-effect dose was 0.2 mg/kg/day in rats and 15 mg/kg/day in rabbits. These no-effect levels are approximately 4 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET. Rosiglitazone reduced the number of uterine implantations and live offspring when juvenile female rats were treated at 40 mg/kg/day from 27 days of age through to sexual maturity (approximately 68 times human AUC at the maximum recommended daily dose). The no-effect level was 2 mg/kg/day (approximately 4 times human AUC at the maximum recommended daily dose). There was no effect on pre- or post-natal survival or growth.
Metformin hydrochloride:
Metformin was not teratogenic in rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 and 6 times the maximum recommended human daily dose of 2,000 mg based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
Labor and Delivery
The effect of AVANDAMET or its components on labor and delivery in humans is unknown.
Nursing Mothers
No studies have been conducted with the combined components of AVANDAMET. In studies performed with the individual components, both rosiglitazone-related material and metformin were detectable in milk from lactating rats. It is not known whether rosiglitazone and/or metformin is excreted in human milk. Because many drugs are excreted in human milk, AVANDAMET should not be administered to a nursing woman. If AVANDAMET is discontinued, and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatric Use
Safety and effectiveness of AVANDAMET in pediatric patients have not been established. AVANDAMET and rosiglitazone are not indicated for use in pediatric patients.
Geriatric Use
Metformin is known to be substantially excreted by the kidney and because the risk of serious adverse reactions to the drug is greater in patients with impaired renal function, AVANDAMET should only be used in patients with normal renal function (see CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY, Pharmacokinetics). Because reduced renal function is associated with increasing age, AVANDAMET should be used with caution in elderly patients. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. Generally, elderly patients should not be titrated to the maximum dose of AVANDAMET (see also WARNINGS and DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
The incidence and types of adverse events reported in a controlled, 32-week double-blind clinical trial of AVANDAMET in drug-naïve patients (n = 468) are shown in Table 8.
|
AVANDAMET |
Metformin |
Rosiglitazone |
|
|
N = 155 |
N = 154 |
N = 159 |
|
|
Preferred term |
% |
% |
% |
|
Nausea/vomiting |
16 |
13 |
8 |
|
Diarrhea |
14 |
21 |
7 |
|
Headache |
11 |
12 |
10 |
|
Dyspepsia |
10 |
8 |
9 |
|
Upper respiratory tract infection |
9 |
7 |
8 |
|
Dizziness |
8 |
3 |
5 |
|
Edema |
6 |
3 |
7 |
|
Nasopharyngitis |
6 |
5 |
4 |
|
Abdominal pain |
5 |
6 |
7 |
|
Arthralgia |
5 |
3 |
7 |
|
Loose Stools |
5 |
6 |
1 |
|
Constipation |
5 |
4 |
6 |
|
Influenza |
1 |
2 |
6 |
The incidence and types of adverse events reported in controlled, 26-week clinical trials of rosiglitazone maleate administered in combination with metformin hydrochloride 2,500 mg/day in comparison to adverse reactions reported in association with rosiglitazone and metformin monotherapies are shown in Table 9. Overall, the types of adverse experiences reported when rosiglitazone was used in combination with metformin were similar to those reported during monotherapy with rosiglitazone.
|
Rosiglitazone |
Placebo |
Metformin |
Rosiglitazone plus metformin |
|
|
N = 2,526 |
N = 601 |
N = 225 |
N = 338 |
|
|
Preferred term |
% |
% |
% |
% |
|
Upper respiratory tract infection |
9.9 |
8.7 |
8.9 |
16.0 |
|
Injury |
7.6 |
4.3 |
7.6 |
8.0 |
|
Headache |
5.9 |
5.0 |
8.9 |
6.5 |
|
Back pain |
4.0 |
3.8 |
4.0 |
5.0 |
|
Hyperglycemia |
3.9 |
5.7 |
4.4 |
2.1 |
|
Fatigue |
3.6 |
5.0 |
4.0 |
5.9 |
|
Sinusitis |
3.2 |
4.5 |
5.3 |
6.2 |
|
Diarrhea |
2.3 |
3.3 |
15.6 |
12.7 |
|
Viral infection |
3.2 |
4.0 |
3.6 |
5.0 |
|
Arthralgia |
3.0 |
4.0 |
2.2 |
5.0 |
|
Anemia |
1.9 |
0.7 |
2.2 |
7.1 |
In the double-blind trial evaluating AVANDAMET in drug-naïve patients, mild (no intervention required) to moderate (minor intervention required) symptomatic hypoglycemia was reported by 18/155 (12%) of patients treated with AVANDAMET, 14/154 (9%) with metformin, and 13/159 (8%) with rosiglitazone. Approximately half of these episodes were accompanied by a simultaneous capillary glucose measurement, and the rate of confirmed hypoglycemia (blood glucose≤50 mg/dL) was low in this clinical study: 0.6% (1/155) for AVANDAMET, 1.3% (2/154) for metformin and 0% with rosiglitazone. No hypoglycemic episode led to withdrawal with AVANDAMET treatment, and no patients required medical intervention due to hypoglycemia.
Reports of hypoglycemia in patients treated with rosiglitazone added to maximum metformin therapy in double-blind studies were more frequent (3.0%) than in patients treated with rosiglitazone (0.6%) or metformin monotherapies (1.3%) or placebo (0.2%). Overall, anemia and edema were generally mild to moderate in severity and usually did not require discontinuation of treatment with rosiglitazone.
In the double-blind trial in drug-naïve patients, the incidence of edema was 6% on AVANDAMET compared to 7% on rosiglitazone and 3% on metformin.
In the double-blind trial in drug-naïve patients, the incidence of anemia was 4% in patients treated with AVANDAMET compared to either rosiglitazone (2%) or metformin (0%). Reports of anemia (7.1%) were greater in patients treated with rosiglitazone added to metformin compared to monotherapy with rosiglitazone. Lower pre-treatment hemoglobin/hematocrit levels in patients enrolled in the metformin and rosiglitazone combination therapy clinical trials may have contributed to the higher reporting rate of anemia in these studies (see ADVERSE REACTIONS, Laboratory Abnormalities, Hematologic).
Edema was reported in 4.8% of patients receiving rosiglitazone compared to 1.3% on placebo, and 2.2% on metformin monotherapy and 4.4% on rosiglitazone in combination with maximum doses of metformin.
Combination with Insulin
The safety profile for AVANDAMET plus insulin was consistent with that of the individual components (rosiglitazone or metformin) and with that of rosiglitazone used in combination with insulin.
The incidence of hypoglycemia (confirmed by fingerstick blood glucose concentration ≤50 mg/dL) was 14% for patients on AVANDAMET plus insulin compared to 10% for patients on insulin monotherapy.
The incidence of edema was 7% when insulin was added to AVANDAMET compared to 3% with insulin monotherapy. This trial excluded patients with pre-existing heart failure or new or worsening edema on AVANDAMET therapy.
However, in 26-week double-blind, fixed-dose studies of rosiglitazone added to insulin, edema was reported with higher frequency (rosiglitazone in combination with insulin, 14.7%; insulin, 5.4%). Reports of new-onset or exacerbation of congestive heart failure occurred at rates of 1% for insulin alone, and 2% (4 mg) and 3% (8 mg) for insulin in combination with rosiglitazone. There were too few events to confirm a dose relationship; however, the incidence of heart failure appeared higher with rosiglitazone 8 mg daily (see BOXED WARNING and WARNINGS, Rosiglitazone maleate).
The incidence of anemia was 2% for AVANDAMET in combination with insulin compared to 1% for insulin monotherapy.
Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the events described below have been identified during post-approval use of AVANDAMET or its individual components. Because these events are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or to always establish a causal relationship to drug exposure.
In postmarketing experience in patients receiving thiazolidinedione therapy, serious adverse events with or without a fatal outcome, potentially related to volume expansion (e.g., heart failure, pulmonary edema, and pleural effusions) have been reported (see BOXED WARNING and WARNINGS, Rosiglitazone maleate).
Rash, pruritus, urticaria, angioedema, anaphylactic reaction, and Stevens-Johnson syndrome have been reported rarely.
Reports of new onset or worsening diabetic macular edema with decreased visual acuity have also been received (see PRECAUTIONS, Rosiglitazone maleate, Macular Edema).
(See also GLUCOPHAGE prescribing information, ADVERSE REACTIONS.)
Laboratory Abnormalities
Hematologic
Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with rosiglitazone maleate (mean decreases in individual studies up to 1.0 gram/dL hemoglobin and up to 3.3% hematocrit). The time course and magnitude of decreases were similar in patients treated with a combination of rosiglitazone and other hypoglycemic agents or rosiglitazone monotherapy. Pre-treatment levels of hemoglobin and hematocrit were lower in patients in metformin combination studies and may have contributed to the higher reporting rate of anemia. In a single study in pediatric patients, decreases in hemoglobin and hematocrit (mean decreases of 0.29 g/dL and 0.95%, respectively) were reported with rosiglitazone. White blood cell counts also decreased slightly in adult patients treated with rosiglitazone. Decreases in hematologic parameters may be related to increased plasma volume observed with rosiglitazone treatment.
In controlled clinical trials of metformin hydrochloride of 29 weeks’ duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such a decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation.
Lipids
Changes in serum lipids have been observed following treatment with rosiglitazone maleate in adults (see CLINICAL STUDIES). Small changes in serum lipid parameters were reported in children treated with rosiglitazone for 24 weeks.
Serum Transaminase Levels
In clinical studies in 4,598 patients treated with rosiglitazone maleate encompassing approximately 3,600 patient years of exposure, there was no evidence of drug-induced hepatotoxicity or elevated ALT levels.
In controlled trials, 0.2% of patients treated with rosiglitazone maleate had reversible elevations in ALT >3X the upper limit of normal compared to 0.2% on placebo and 0.5% on active comparators. Hyperbilirubinemia was found in 0.3% of patients treated with rosiglitazone compared with 0.9% treated with placebo and 1% in patients treated with active comparators.
In the clinical program including long-term, open-label experience, the rate per 100 patient years of exposure of ALT increase to >3X the upper limit of normal was 0.35 for patients treated with rosiglitazone maleate, 0.59 for placebo-treated patients, and 0.78 for patients treated with active comparator agents.
In pre-approval clinical trials, there were no cases of idiosyncratic drug reactions leading to hepatic failure. In postmarketing experience with rosiglitazone maleate, reports of hepatic enzyme elevations 3 or more times the upper limit of normal and hepatitis have been received (see PRECAUTIONS, Hepatic Effects).
OVERDOSAGE
Rosiglitazone maleate
Limited data are available with regard to overdosage in humans. In clinical studies in volunteers, rosiglitazone has been administered at single oral doses of up to 20 mg and was well tolerated. In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical status.
Metformin hydrochloride
Hypoglycemia has not been seen with ingestion of up to 85 grams of metformin hydrochloride, although lactic acidosis has occurred in such circumstances (see WARNINGS). Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated metformin from patients in whom metformin overdosage is suspected.
DOSAGE AND ADMINISTRATION
General
The dosage of antidiabetic therapy with AVANDAMET should be individualized on the basis of effectiveness and tolerability while not exceeding the maximum recommended daily dose of 8 mg/2,000 mg. The risk-benefit of initiating monotherapy versus dual therapy with AVANDAMET should be considered. (See CLINICAL TRIALS, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.)
All patients should start the rosiglitazone component of AVANDAMET at the lowest recommended dose. Further increases in the dose of rosiglitazone should be accompanied by careful monitoring for adverse events related to fluid retention (see BOXED WARNING and WARNINGS, Rosiglitazone maleate).
AVANDAMET is generally given in divided doses with meals, with gradual dose escalation. This reduces gastrointestinal side effects (largely due to metformin) and permits determination of the minimum effective dose for the individual patient.
Sufficient time should be given to assess adequacy of therapeutic response. Fasting plasma glucose (FPG) should be used to determine the therapeutic response to AVANDAMET.
AVANDAMET in Drug-Naїve Patients
The recommended starting dose of AVANDAMET is 2 mg/500 mg administered once or twice daily. For patients with HbA1c >11% or FPG >270 mg/dL, a starting dose of 2 mg/500 mg twice daily may be considered. The dose of AVANDAMET may be increased in increments of 2 mg/500 mg per day to a maximum of 8 mg/2,000 mg per day given in divided doses if patients are not adequately controlled after 4 weeks.
AVANDAMET in Patients Inadequately Controlled with Rosiglitazone or Metformin Monotherapy
The selection of the dose of AVANDAMET in patients treated with rosiglitazone and/or metformin therapy should be based on the patient’s current doses of rosiglitazone and/or metformin. After an increase in metformin dosage, dose titration is recommended if patients are not adequately controlled after 1 to 2 weeks. After an increase in rosiglitazone dosage, dose titration is recommended if patients are not adequately controlled after 8 to 12 weeks.
For patients inadequately controlled on metformin monotherapy, the usual starting dose of AVANDAMET is 4 mg rosiglitazone (total daily dose) plus the dose of metformin already being taken (see Table 10).
For patients inadequately controlled on rosiglitazone monotherapy, the usual starting dose of AVANDAMET is 1,000 mg metformin (total daily dose) plus the dose of rosiglitazone already being taken (see Table 10).
When switching from combination therapy of rosiglitazone plus metformin as separate tablets, the usual starting dose of AVANDAMET is the dose of rosiglitazone and metformin already being taken.
If additional glycemic control is needed, the daily dose of AVANDAMET may be increased by increments of 4 mg rosiglitazone and/or 500 mg metformin, up to the maximum recommended total daily dose of 8 mg/2,000 mg.
|
PRIOR THERAPY |
Usual AVANDAMET Starting Dose |
|
|
Total daily dose |
Tablet strength |
Number of tablets |
|
Metformin HCl* | ||
|
1,000 mg/day |
2 mg/500 mg |
1 tablet twice a day |
|
2,000 mg/day |
2 mg/1,000 mg |
1 tablet twice a day |
|
Rosiglitazone | ||
|
4 mg/day |
2 mg/500 mg |
1 tablet twice a day |
|
8 mg/day |
4 mg/500 mg |
1 tablet twice a day |
|
* For patients on doses of metformin HCl between 1,000 and 2,000 mg/day, initiation of AVANDAMET requires individualization of therapy. |
||
Specific Patient Populations
Pregnancy
AVANDAMET is not recommended for use in pregnancy.
Geriatric
The initial and maintenance dosing of AVANDAMET should be conservative in patients with advanced age, due to the potential for decreased renal function in this population.
Renal Impairment
Any dosage adjustment should be based on a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of AVANDAMET. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly (see WARNINGS).
Hepatic Impairment
Therapy with AVANDAMET should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X upper limit of normal at start of therapy) (see PRECAUTIONS, Hepatic Effects and CLINICAL PHARMACOLOGY, Special Populations, Hepatic Impairment). Liver enzyme monitoring is recommended in all patients prior to initiation of therapy with AVANDAMET and periodically thereafter (see PRECAUTIONS, Hepatic Effects).
Pediatric
Safety and effectiveness of AVANDAMET in pediatric patients have not been established. AVANDAMET and rosiglitazone are not indicated for use in pediatric patients.
HOW SUPPLIED
Tablets: Each tablet contains rosiglitazone as the maleate and metformin hydrochloride as follows:
2 mg/500 mg – pale pink, film-coated oval tablet, debossed with gsk on one side and 2/500 on the other.
4 mg/500 mg – orange, film-coated oval tablet, debossed with gsk on one side and 4/500 on the other.
2 mg/1,000 mg – yellow, film-coated oval tablet, debossed with gsk on one side and 2/1000 on the other.
4 mg/1,000 mg – pink, film-coated oval tablet, debossed with gsk on one side and 4/1000 on the other.
2 mg/500 mg bottles of 60: NDC 0007-3167-18
4 mg/500 mg bottles of 60: NDC 0007-3168-18
2 mg/1,000 mg bottles of 60: NDC 0007-3163-18
4 mg/1,000 mg bottles of 60: NDC 0007-3164-18
STORAGE
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Dispense in a tight, light-resistant container.
REFERENCE
-
Park JY, Kim KA, Kang MH, et al. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther 2004;75:157-162.
GLUCOPHAGE is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
AVANDAMET is a registered trademark of GlaxoSmithKline.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2007, GlaxoSmithKline. All rights reserved.
September 2007 AVM:17PI
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __ _ _ _ _ _ _ _
PATIENT INFORMATION
AVANDAMET®(ah-VAN-duh-met)
Rosiglitazone Maleate and Metformin Hydrochloride Tablets
Read this information carefully before you start taking AVANDAMET and read the information you get each time you get more AVANDAMET. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about AVANDAMET, ask your doctor. Only your doctor can determine if AVANDAMET is right for you.
What is AVANDAMET?
AVANDAMET is a medicine used, along with diet and exercise, to treat type 2 (“adult-onset”) diabetes (“high sugar”). This is also called non-insulin-dependent diabetes mellitus. People who have type 2 diabetes do not make enough insulin or do not respond normally to the insulin their bodies make. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems including kidney damage, amputation and blindness. Diabetes is also closely linked to heart disease. The main goal of treating diabetes is to lower your blood sugar to a normal level. Studies have shown that controlling blood sugar may help prevent or delay complications of diabetes such as heart disease, kidney disease or blindness.
High blood sugar can be lowered by diet and exercise, by a number of medicines taken by mouth, and by insulin shots. AVANDAMET combines two sugar- (glucose) lowering medicines, rosiglitazone and metformin, in one tablet. Metformin works mainly by decreasing the production of sugar by your liver. Rosiglitazone helps your body respond better to its natural insulin and does not cause your body to make more insulin. These medicines work together to help control your blood sugar.
Before you take AVANDAMET, you should first try to control your diabetes by diet and exercise. In order for AVANDAMET to be most effective, you should continue to exercise and follow the diet recommended for your diabetes even while taking AVANDAMET.
WARNING: A small number of people who have taken metformin, one of the two drugs that make up AVANDAMET, have developed a serious condition called lactic acidosis. Lactic acidosis is caused by a build-up of lactic acid in the blood. Lactic acidosis happens most often in people with kidney problems. People with kidney problems should not take AVANDAMET. (See “What are the side effects of AVANDAMET?”)
Who should not take AVANDAMET?
Do not take AVANDAMET if you:
-
Had liver problems while taking REZULIN® (troglitazone), another medicine for diabetes.
-
Have kidney or liver problems. Before you take AVANDAMET and while you take it, your doctor should test your blood to check for signs of kidney or liver problems.
-
Have heart failure, until you talk with your doctor. Certain patients with heart failure should not start taking AVANDAMET. Rosiglitazone, one drug in AVANDAMET, may cause fluid retention (swelling or edema), alone or in combination with other diabetes medicines. Fluid retention can lead to heart failure or make heart failure worse. Call your doctor if you have shortness of breath or a sudden weight change.
-
Drink a lot of alcohol (all the time or short binge drinking).
-
Are seriously dehydrated (as when your body has lost a lot of water from diarrhea or vomiting).
-
Are going to have an x-ray procedure with an injection of dyes (contrast agents) in your vein with a needle. Talk to your doctor about when to stop AVANDAMET and when to start it again.
-
Are scheduled to have surgery or an operation. Talk to your doctor about when to stop AVANDAMET and when to start it again.
-
Develop a serious condition such as a heart attack, severe infection, or a stroke.
-
Are 80 years or older and have NOT had your kidney function tested.
-
Have had an allergic reaction to AVANDIA® (rosiglitazone) or metformin (e.g., GLUCOPHAGE®).
-
Have type 1 (“juvenile”) diabetes or a history of metabolic ketoacidosis, including diabetic ketoacidosis.
-
Have a type of diabetic eye disease called macular edema (swelling of the back of the eye) until you talk to your doctor.
Some conditions increase your chance of getting lactic acidosis, or cause other problems if you take AVANDAMET. Your risk of getting lactic acidosis is very low as long as your kidneys and liver are healthy. Most of the conditions listed above can increase your chance of getting lactic acidosis or cause other problems if you take AVANDAMET.
Can AVANDAMET be used in nursing or pregnant women or in children?
Tell your doctor if you are pregnant, plan to become pregnant, or if you are nursing a child. If you are a premenopausal woman (before the “change of life”), who is not having periods regularly or at all, you may need to consider birth controloptions since AVANDAMET may increase your chances of becoming pregnant. Talk with your doctor about your choices.
AVANDAMET has not been studied in children. AVANDAMET is not recommended for use in children.
How should I take AVANDAMET?
AVANDAMET should be taken by mouth and with meals. AVANDAMET should be taken twice a day to help improve blood sugar levels. Your doctor may need to adjust your dose until your blood sugar is better controlled.
While you are taking AVANDAMET, you should also follow recommendations of your healthcare professional for appropriate diet and exercise. Diet and exercise can help your body use blood sugar better. Test your blood sugar regularly as your doctor tells you.
AVANDAMET can begin to work 1 or 2 weeks after you start taking it. It may take 2-3 months to see the full effect.
Your doctor should check your eyes regularly. Very rarely, some patients have experienced vision changes due to swelling in the back of the eye while taking rosiglitazone, one of the drugs in AVANDAMET.
While taking AVANDAMET tell your doctor if you:
-
Have an illness that causes severe vomiting, diarrhea or fever, or if you drink a much lower amount of liquid than normal. These conditions can lead to severe dehydration (loss of water from your body). You may need to stop taking AVANDAMET for a short time.
-
Plan to have surgery or an x-ray procedure with injection of dye (contrast agent). You may need to stop taking AVANDAMET for a short time.
-
Start to take other medicines (including non-prescription and dietary or herbal supplements) or change how you take a medicine. AVANDAMET may affect how well other drugs work, and some drugs may affect how well AVANDAMET works. Some medicines may cause high blood sugar or low blood sugar.
What should I avoid while taking AVANDAMET?
Avoid drinking a lot of alcoholic drinks while taking AVANDAMET. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Drinking a lot of alcohol can increase the chance of getting lactic acidosis.
What are the possible side effects of AVANDAMET?
In rare cases, metformin, one of the drugs in AVANDAMET, can cause a serious side effect called lactic acidosis. This is caused by a build-up of lactic acid in your blood. This build-up can cause serious damage. Lactic acidosis is a medical emergency that must be treated in a hospital. Lactic acidosis is rare and has occurred mostly in people whose kidneys were not working normally. Lactic acidosis has been reported in about 1 in 33,000 patients taking metformin over the course of a year. Although rare, if lactic acidosis does occur, it can be fatal in up to half the people who develop it. Call your doctor right away if you have signs of lactic acidosis such as:
-
feeling very weak, tired, or uncomfortable (malaise)
-
unusual muscle pain
-
unusual sleepiness
-
rapid breathing that you can’t explain
-
unusual or unexpected stomach problems (such as nausea or vomiting)
-
low body temperature
-
feeling dizzy or light-headed
-
suddenly having a slow or uneven heartbeat
It is important for your liver to be working normally when you take AVANDAMET. Your liver helps remove lactic acid from your blood. Before you take AVANDAMET, your doctor will test your blood to check for signs of liver problems. Sometimes after you start taking it, your doctor may recheck your blood. Very rarely, serious liver problems have been reported with rosiglitazone, one of the drugs in AVANDAMET. Call your doctor right away if you have unexplained symptoms such as:
-
nausea or vomiting
-
stomach pain
-
unusual or unexplained tiredness
-
loss of appetite
-
dark urine
-
yellowing of your skin or the whites of your eyes
AVANDAMET may cause fluid retention or swelling, which could lead to heart failure or make heart failure worse, so tell your doctor if you have a history of heart failure or swelling (edema). Call your doctor right away if you have symptoms such as:
-
swelling or fluid retention, especially of the ankles or legs
-
shortness of breath or trouble breathing, especially when you lie down
-
unusual tiredness
-
an unusually rapid increase in weight
There is a small risk of developing low blood sugar (hypoglycemia) while taking AVANDAMET. Lightheadedness, dizziness, shakiness or hunger may indicate that your blood sugar is too low. This can happen if you skip meals, if you use another medicine that lowers blood sugar, or if you have certain medical problems.
Fractures, usually in the hand, upper arm or foot, have occurred in females taking rosiglitazone, one of the components of AVANDAMET. Talk to your doctor for advice on how to keep your bones healthy.
Other Side Effects. Common side effects of AVANDAMET are diarrhea, nausea, and upset stomach. These side effects usually occur during the first few weeks of therapy. Taking AVANDAMET with meals can help reduce these side effects. Stomach problems when you first take AVANDAMET are common. However, stomach problems that start up later may be a sign of something more serious and should be discussed with your doctor. Other common side effects are cold-like symptoms, headache, weight gain, and anemia.
How should I store AVANDAMET?
AVANDAMET should be stored at room temperature in a childproof container out of the reach of children. Store AVANDAMET in its original container.
General Advice about prescription medicines
This leaflet summarizes important information about AVANDAMET. If you have questions or problems, talk with your doctor or other healthcare provider. You can ask your doctor or pharmacist for information about AVANDAMET that is written for healthcare providers. Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use AVANDAMET for a condition for which it was not prescribed. Do not share your medicine with other people.
AVANDAMET and AVANDIA are registered trademarks of GlaxoSmithKline.
GLUCOPHAGE is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
REZULIN is a registered trademark of Parke-Davis Pharmaceuticals Ltd.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2007, GlaxoSmithKline. All rights reserved.
September 2007 AVM:17PIL
| AVANDAMET (rosiglitazone maleate and metformin hydrochloride) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| AVANDAMET (rosiglitazone maleate and metformin hydrochloride) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| AVANDAMET (rosiglitazone maleate and metformin hydrochloride) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| AVANDAMET (rosiglitazone maleate and metformin hydrochloride) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 09/2007GlaxoSmithKline