ORACEA
-
doxycycline capsule, delayed release pellets
Galderma Laboratories, L.P.
----------
Rx Only
KEEP OUT OF REACH OF CHILDREN
The dosage of ORACEA differs from that of doxycycline used to treat infections. To reduce the development of resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ORACEA should be used only as indicated.
ORACEA is indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients.
This formulation of doxycycline has not been evaluated as an antibacterial in the treatment of infections.
DESCRIPTION
ORACEA (doxycycline, USP) capsules 40 mg are hard gelatin capsule shells filled with two types of doxycycline beads (30 mg immediate release and 10 mg delayed release) that together provide a dose of 40 mg of anhydrous doxycycline (C22H24N2O8).
The structural formula of doxycycline, USP is:
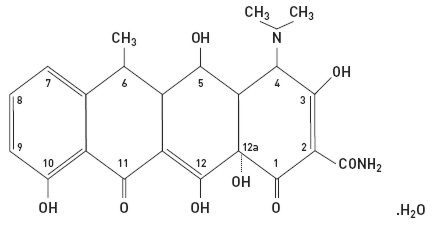
with an empirical formula of C22H24N2O8•H2O and a molecular weight of 462.46. The chemical designation for doxycycline is 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, [4S-(4aα, 4aα, 5α, 5aα, 6a,12aα)]-, monohydrate. It is very slightly soluble in water.
Inert ingredients in the formulation are: hypromellose, iron oxide red, iron oxide yellow, methacrylic acid copolymer, polyethylene glycol, Polysorbate 80, sugar spheres, talc, titanium dioxide, and triethyl citrate. Active ingredients: Each capsule contains doxycycline, USP in an amount equivalent to 40 mg of anhydrous doxycycline.
CLINICAL PHARMACOLOGY
Pharmacokinetics
ORACEA capsules are not bioequivalent to other doxycycline products. The pharmacokinetics of doxycycline following oral administration of ORACEA was investigated in 2 volunteer studies involving 61 adults. Pharmacokinetic parameters for ORACEA following single oral doses and at steady-state in healthy subjects are presented in Table 1.
| N | Cmax*
(ng/mL) | Tmax †
(hr) | AUC0-∞*
(ng∙hr/mL) | t1/2 (hr)* |
|
|---|---|---|---|---|---|
| Single Dose 40 mg capsules | 30 | 510 ± 220.7 | 3.00 (1.0-4.1) | 9227 ± 3212.8 | 21.2 ± 7.6 |
| Steady-State‡
40 mg capsules | 31 | 600 ± 194.2 | 2.00 (1.0-4.0) | 7543 ± 2443.9 | 23.2 ± 6.2 |
Absorption
In a single-dose food-effect study involving administration of ORACEA to healthy volunteers, concomitant administration with a 1000 calorie, high-fat, high-protein meal that included dairy products, resulted in a decrease in the rate and extent of absorption (Cmax and AUC) by about 45% and 22%, respectively, compared to dosing under fasted conditions. This decrease in systemic exposure can be clinically significant, and therefore if ORACEA is taken close to meal times, it is recommended that it be taken at least one hour prior to or two hours after meals.
Metabolism
Major metabolites of doxycycline have not been identified. However, enzyme inducers such as barbiturates, carbamazepine, and phenytoin decrease the half-life of doxycycline.
Excretion
Doxycycline is excreted in the urine and feces as unchanged drug. It is reported that between 29% and 55.4% of an administered dose can be accounted for in the urine by 72 hours. Terminal half-life averaged 21.2 hours in subjects receiving a single dose of ORACEA.
Special Populations
Pediatric
Doxycycline pharmacokinetics have not been evaluated in pediatric patients (see WARNINGS section).
Gender
The pharmacokinetics of ORACEA were compared in 16 male and 14 female subjects under fed and fasted conditions. While female subjects had a higher Cmax and AUC than male subjects, these differences were thought to be due to differences in body weight/lean body mass.
Renal Insufficiency
Studies have shown no significant difference in serum half-life of doxycycline in patients with normal and severely impaired renal function. Hemodialysis does not alter the serum half-life of doxycycline.
Hepatic Insufficiency
Doxycycline pharmacokinetics have not been evaluated in patients with hepatic insufficiency.
MICROBIOLOGY
Doxycycline is a member of the tetracycline class of antibacterial drugs. The plasma concentrations of doxycycline achieved with ORACEA during administration (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION) are less than the concentration required to treat bacterial diseases. In vivo microbiological studies utilizing a similar drug exposure for up to 18 months demonstrated no detectable long-term effects on bacterial flora of the oral cavity, skin, intestinal tract, and vagina.
ORACEA should not be used for treating bacterial infections, providing antibacterial prophylaxis, or reducing the numbers or eliminating microorganisms associated with any bacterial disease.
CLINICAL STUDIES
The safety and efficacy of ORACEA in the treatment of only inflammatory lesions (papules and pustules) of rosacea was evaluated in two randomized, placebo-controlled, multi-centered, double-blind, 16-week Phase 3 studies involving 537 patients (total of 269 patients on ORACEA from the two studies) with rosacea (10 to 40 papules and pustules and two or fewer nodules). Pregnant and nursing women, patients <18 years of age, and patients with ocular rosacea and/or blepharitis/meibomianitis who require ophthalmologic treatment were excluded from study. Mean baseline lesion counts were 20 and 21 for ORACEA and placebo patient groups respectively.
At Week 16, patients in the ORACEA group were evaluated using co-primary endpoints of mean reduction in lesion counts and a dichotomized static Investigator's Global Assessment of Clear or Almost Clear (defined as 1 to 2 small papules or pustules) when compared to the placebo group in both Phase 3 studies.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| ORACEA 40 mg N = 127 | Placebo N = 124 | ORACEA 40 mg N = 142 | Placebo N = 144 |
|
|
||||
| Mean Change in Lesion Count from Baseline | -11.8 | -5.9 | -9.5 | -4.3 |
| No. (%) of Subjects Clear or Almost Clear in the IGA* | 39 (30.7%) | 24 (19.4%) | 21 (14.8%) | 9 (6.3%) |
Patients treated with ORACEA did not demonstrate significant improvement in erythema when compared to those treated with placebo.
INDICATIONS AND USAGE
ORACEA is indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. No meaningful effect was demonstrated for generalized erythema (redness) of rosacea. ORACEA has not been evaluated for the treatment of the erythematous, telangiectatic, or ocular components of rosacea. Efficacy of ORACEA beyond 16 weeks and safety beyond 9 months have not been established.
This formulation of doxycycline has not been evaluated in the treatment or prevention of infections. ORACEA should not be used for treating bacterial infections, providing antibacterial prophylaxis, or reducing the numbers or eliminating microorganisms associated with any bacterial disease.
To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ORACEA should be used only as indicated.
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to doxycycline or any of the other tetracyclines.
WARNINGS
Teratogenic effects
- 1)
-
Doxycycline, like other tetracycline-class antibiotics, can cause fetal harm when administered to a pregnant woman. If any tetracycline is used during pregnancy or if the patient becomes pregnant while taking these drugs, the patient should be informed of the potential hazard to the fetus and treatment stopped immediately.
ORACEA should not be used during pregnancy (see PRECAUTIONS: Pregnancy). - 2)
- The use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the drug but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. Tetracycline drugs, therefore, should not be used during tooth development unless other drugs are not likely to be effective or are contraindicated.
- 3)
- All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development on the developing fetus. Evidence of embryotoxicity has been noted in animals treated early in pregnancy (see PRECAUTIONS: Pregnancy section).
Gastrointestinal effects
Pseudomembranous colitis has been reported with nearly all antibacterial agents and may range from mild to life-threatening.
Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated colitis".
If a diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
Metabolic effects
The anti-anabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline-class antibiotics may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulations of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated, and if therapy is prolonged, serum level determinations of the drug may be advisable.
Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Although this was not observed during the duration of the clinical studies with ORACEA, patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using ORACEA. If patients need to be outdoors while using ORACEA, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician.
PRECAUTIONS
General
Safety of ORACEA beyond 9 months has not been established.
As with other antibiotic preparations, use of ORACEA may result in overgrowth of non-susceptible microorganisms, including fungi. If superinfection occurs, ORACEA should be discontinued and appropriate therapy instituted. Although not observed in clinical trials with ORACEA, the use of tetracyclines may increase the incidence of vaginal candidiasis.
ORACEA should be used with caution in patients with a history of or predisposition to candidiasis overgrowth.
Bacterial resistance to tetracyclines may develop in patients using ORACEA. Because of the potential for drug-resistant bacteria to develop during the use of ORACEA, it should be used only as indicated.
Autoimmune Syndromes
Tetracyclines have been associated with the development of autoimmune syndromes. Symptoms may be manifested by fever, rash, arthralgia, and malaise. In symptomatic patients, liver function tests, ANA, CBC, and other appropriate tests should be performed to evaluate the patients. Use of all tetracycline-class drugs should be discontinued immediately.
Tissue Hyperpigmentation
Tetracycline class antibiotics are known to cause hyperpigmentation. Tetracycline therapy may induce hyperpigmentation in many organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. Skin and oral pigmentation has been reported to occur independently of time or amount of drug administration, whereas other pigmentation has been reported to occur upon prolonged administration. Skin pigmentation includes diffuse pigmentation as well as over sites of scars or injury.
Pseudotumor cerebri
Bulging fontanels in infants and benign intracranial hypertension in adults have been reported in individuals receiving tetracyclines. These conditions disappeared when the drug was discontinued.
Information for Patients
See Patient Package Insert that accompanies this Package Insert for additional information to give patients.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including doxycycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using doxycycline. If patients need to be outdoors while using doxycycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of sunburn.
- Concurrent use of doxycycline may render oral contraceptives less effective (see Drug Interactions).
- Autoimmune syndromes, including drug-induced lupus-like syndrome, autoimmune hepatitis, vasculitis and serum sickness have been observed with tetracycline-class antibiotics, including doxycycline. Symptoms may be manifested by arthralgia, fever, rash and malaise. Patients who experience such symptoms should be cautioned to stop the drug immediately and seek medical help.
- Patients should be counseled about discoloration of skin, scars, teeth or gums that can arise from doxycycline therapy.
- Take ORACEA exactly as directed. Increasing doses beyond 40 mg every morning may increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- It is recommended that ORACEA not be used by pregnant or breast feeding women. (see Carcinogenesis, Mutagenesis, Impairment of Fertility, Pregnancy and Nursing Mothers sections).
- It is recommended that ORACEA not be used by individuals of either gender who are attempting to conceive a child (see Carcinogenesis, Mutagenesis, Impairment of Fertility, and Pregnancy sections).
Laboratory Tests
Periodic laboratory evaluations of organ systems, including hematopoietic, renal and hepatic studies should be performed. Appropriate tests for autoimmune syndromes should be performed as indicated.
Drug Interactions
- Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
- Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
- The concurrent use of tetracycline and methoxyflurane has been reported to result in fatal renal toxicity.
- Absorption of tetracyclines is impaired by bismuth subsalicylate, proton pump inhibitors, antacids containing aluminum, calcium or magnesium and iron-containing preparations.
- Doxycycline may interfere with the effectiveness of low dose oral contraceptives. To avoid contraceptive failure, females are advised to use a second form of contraceptive during treatment with doxycycline.
- There have been reports of pseudotumor cerebri (benign intracranial hypertension) associated with the concomitant use of isotretinoin and tetracyclines. Since both oral retinoids, including isotretinoin and acitretin, and the tetracyclines, primarily minocycline, can cause increased intracranial pressure, the concurrent use of an oral retinoid and a tetracycline should be avoided.
Drug/Laboratory Test Interactions
False elevations of urinary catecholamine levels may occur due to interference with the fluorescence test.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Doxycycline was assessed for potential to induce carcinogenesis in a study in which the compound was administered to Sprague-Dawley rats by gavage at dosages of 20, 75, and 200 mg/kg/day for two years. An increased incidence of uterine polyps was observed in female rats that received 200 mg/kg/day, a dosage that resulted in a systemic exposure to doxycycline approximately 12.2 times that observed in female humans who use ORACEA (exposure comparison based upon area under the curve (AUC) values). No impact upon tumor incidence was observed in male rats at 200 mg/kg/day, or in either gender at the other dosages studied. Evidence of oncogenic activity was obtained in studies with related compounds, i.e., oxytetracycline (adrenal and pituitary tumors) and minocycline (thyroid tumors).
Doxycycline demonstrated no potential to cause genetic toxicity in an in vitro point mutation study with mammalian cells (CHO/HGPRT forward mutation assay) or in an in vivo micronucleus assay conducted in CD-1 mice. However, data from an in vitro assay with CHO cells for potential to cause chromosomal aberrations suggest that doxycycline is a weak clastogen.
Oral administration of doxycycline to male and female Sprague-Dawley rats adversely affected fertility and reproductive performance, as evidenced by increased time for mating to occur, reduced sperm motility, velocity, and concentration, abnormal sperm morphology, and increased pre- and post-implantation losses. Doxycycline induced reproductive toxicity at all dosages that were examined in this study, as even the lowest dosage tested (50 mg/kg/day) induced a statistically significant reduction in sperm velocity. Note that 50 mg/kg/day is approximately 3.6 times the amount of doxycycline contained in the recommended daily dose of ORACEA for a 60-kg human when compared on the basis of AUC estimates. Although doxycycline impairs the fertility of rats when administered at sufficient dosage, the effect of ORACEA on human fertility is unknown.
Pregnancy
Teratogenic Effects
Pregnancy Category D
(see WARNINGS section). Results from animal studies indicate that doxycycline crosses the placenta and is found in fetal tissues.
Nursing Mothers
Tetracyclines are excreted in human milk. Because of the potential for serious adverse reactions in infants from doxycycline, ORACEA should not be used in mothers who breastfeed. (see WARNINGS section).
Pediatric Use
ORACEA should not be used in infants and children less than 8 years of age (see WARNINGS section). ORACEA has not been studied in children of any age with regard to safety or efficacy, therefore use in children is not recommended.
ADVERSE REACTIONS
Adverse Reactions in Clinical Trials of ORACEA
In controlled clinical trials of adult patients with mild to moderate rosacea, 537 patients received ORACEA or placebo over a 16-week period. The most frequent adverse reactions occurring in these studies are listed in Table 3.
| ORACEA | Placebo | |
|---|---|---|
| Note: Percentages based on total number of study participants in each treatment group. | ||
| Nasopharyngitis | 13 (4.8) | 9 (3.4) |
| Pharyngolaryngeal Pain | 3 (1.1) | 2 (0.7) |
| Sinusitis | 7 (2.6) | 2 (0.7) |
| Nasal Congestion | 4 (1.5) | 2 (0.7) |
| Fungal Infection | 5 (1.9) | 1 (0.4) |
| Influenza | 5 (1.9) | 3 (1.1) |
| Diarrhea | 12 (4.5) | 7 (2.6) |
| Abdominal Pain Upper | 5 (1.9) | 1 (0.4) |
| Abdominal Distention | 3 (1.1) | 1 (0.4) |
| Abdominal Pain | 3 (1.1) | 1 (0.4) |
| Stomach Discomfort | 3 (1.1) | 2 (0.7) |
| Dry Mouth | 3 (1.1) | 0 (0) |
| Hypertension | 8 (3.0) | 2 (0.7) |
| Blood Pressure Increase | 4 (1.5) | 1 (0.4) |
| Aspartate Aminotransferase Increase | 6 (2.2) | 2 (0.7) |
| Blood Lactate Dehydrogenase Increase | 4 (1.5) | 1 (0.4) |
| Blood Glucose Increase | 3 (1.1) | 0 (0) |
| Anxiety | 4 (1.5) | 0 (0) |
| Pain | 4 (1.5) | 1 (0.4) |
| Back Pain | 3 (1.1) | 0 (0) |
| Sinus Headache | 3 (1.1) | 0 (0) |
Adverse Reactions for Tetracyclines
The following adverse reactions have been observed in patients receiving tetracyclines at higher, antimicrobial doses:
Gastrointestinal: anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, and inflammatory lesions (with vaginal candidiasis) in the anogenital region. Hepatotoxicity has been reported rarely. Rare instances of esophagitis and esophageal ulcerations have been reported in patients receiving the capsule forms of the drugs in the tetracycline class. Most of the patients experiencing esophagitis and/or esophageal ulceration took their medication immediately before lying down. (see DOSAGE AND ADMINISTRATION section).
Skin: maculopapular and erythematous rashes. Exfoliative dermatitis has been reported but is uncommon. Photosensitivity is discussed above. (see WARNINGS section).
Renal toxicity: Rise in BUN has been reported and is apparently dose-related. (see WARNINGS section).
Hypersensitivity reactions: urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, serum sickness, pericarditis, and exacerbation of systemic lupus erythematosus.
Blood: Hemolytic anemia, thrombocytopenia, neutropenia, and eosinophilia have been reported.
OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases of overdose.
DOSAGE AND ADMINISTRATION
THE DOSAGE OF ORACEA DIFFERS FROM THAT OF DOXYCYCLINE USED TO TREAT INFECTIONS. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS INCLUDING THE DEVELOPMENT OF RESISTANT MICROORGANISMS.
One ORACEA Capsule (40 mg) should be taken once daily in the morning on an empty stomach, preferably at least one hour prior to or two hours after meals.
Efficacy beyond 16 weeks and safety beyond 9 months have not been established.
Administration of adequate amounts of fluid along with the capsules is recommended to wash down the capsule to reduce the risk of esophageal irritation and ulceration. (see ADVERSE REACTIONS section).
HOW SUPPLIED
ORACEA (beige opaque capsule printed with CGPI 40) containing doxycycline, USP in an amount equivalent to 40 mg of anhydrous doxycycline. Bottle of 30 (NDC 64682-009-01).
PACKAGE LABEL
NDC 64682-009-01
Oracea®
(doxycycline, USP) Capsules
40 mg*
*30 mg immediate release & 10 mg delayed release beads
CollaGenex
pharmaceuticals
30 Capsules
Rx Only
INERT INGREDIENTS:
Hypromellose, Iron Oxide Red, Iron Oxide Yellow, Methacrylic Acid Copolymer, Polyethylene Glycol, Polysorbate 80, Sugar Spheres, Talc, Titanium Dioxide, and Triethyl Citrate
DOSAGE: ONE CAPSULE PER DAY
Store at controlled room temperature of 15°C - 30°C (59°F - 86°F).
Manufactured for: CollaGenex Pharmaceuticals, Inc.
Newtown, PA 18940
Manufactured by: Cardinal Health, Inc.
Winchester, KY 40391
Keep out of reach of children.
| ORACEA
doxycycline capsule, delayed release pellets |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA050805 | 05/27/2006 | 07/31/2010 |
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| CATALENT PHARMA SOLUTIONS, INC. | 829672745 | MANUFACTURE | |
Revised: 05/2006 Galderma Laboratories, L.P.