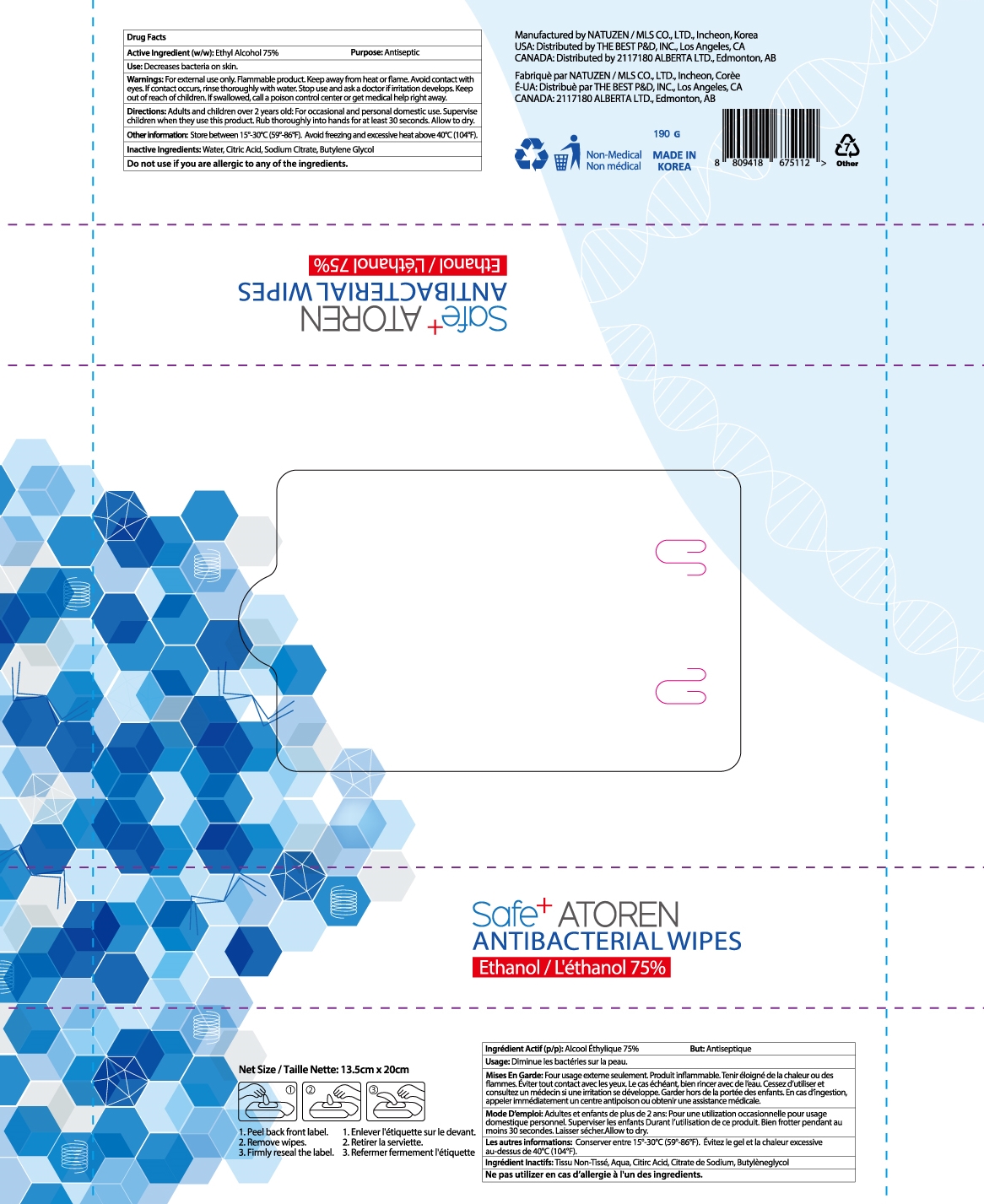
ATOREN ANTIBACTERIAL WIPES- alcohol liquid
MLS
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
73984-334
WARNINGS
Keep out of reach of children. If swallowed, call a poison control center or get medical help right away.
| ATOREN ANTIBACTERIAL WIPES
alcohol liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - MLS (689850283) |
| Registrant - MLS (689850283) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MLS | 689850283 | manufacture(73984-334) | |
Revised: 1/2021
Document Id: b937cf6a-0837-9136-e053-2a95a90ad07c
Set id: a9ed2712-dbe4-3deb-e053-2995a90a0ca3
Version: 2
Effective Time: 20210118
MLS